A kind of preparation method of high-purity fecl2·4h2o
A fecl2·4h2o, high-purity technology, applied in iron halide and other directions, can solve the problems of polluting tap water, complicated treatment methods, and complicated treatment processes, and achieves the effect of improving the scope of industrial applications, increasing economic benefits, and low equipment investment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
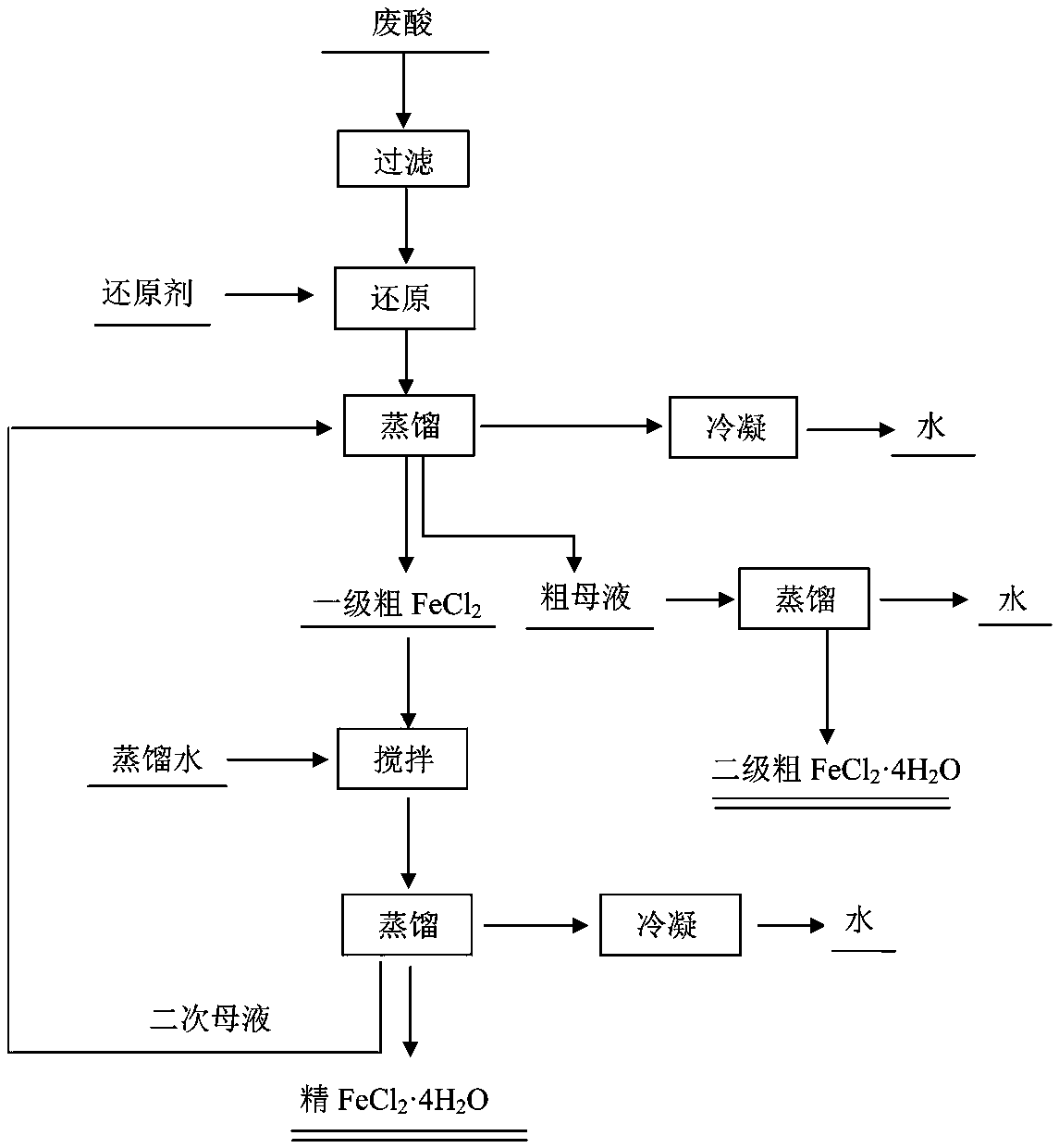
[0020] Such as figure 1 High-purity FeCl shown 2 4H 2 The preparation method of O, with hot-dip galvanizing waste acid as raw material, comprises the following operations:
[0021] 1) Filter the waste hot-dip galvanizing acid to remove suspended matter and solid floating matter;
[0022] 2) Add a reducing agent to the spent acid after step (1) to make Fe 3+ Convert to Fe 2+ ;
[0023] 3) Vacuum distillation of the reduced waste acid to obtain FeCl 2 4H 2 O crystallization and FeCl 2 Mother liquor;
[0024] 4) FeCl obtained in step (3) 2 4H 2 O crystallization and FeCl 2 The first grade FeCl is obtained after the mother liquor is filtered 2 4H 2 O crystallization and mother liquor, and then the mother liquor is thoroughly distilled to obtain secondary FeCl 2 4H 2 O product, the secondary FeCl 2 4H 2 O product as a by-product; the secondary FeCl 2 4H 2 O products can be directly used for sewage and wastewater treatment;
[0025] 5) The primary FeCl 2 4H 2 O...
Embodiment 1
[0038] The chemical analysis of the waste acid of the sample hot-dip galvanizing is as follows: FeCl 2 : 40%, FeCl 3 : 5%, HCl: 0.4%, impurity: 0.3%, and the balance is water.
[0039] Experimental steps:
[0040] (1) Filter the waste acid with a plate and frame filter to remove suspended solids, solid floating solids, etc.
[0041] (2) Add a certain amount of reducing agent (the reducing agent is iron slag) to the waste acid filtered by the plate and frame filter to make Fe 3+ Convert to Fe 2+ . The weight ratio of reducing agent to waste acid is 1:40.
[0042] (3) Low-temperature vacuum distillation of the obtained waste acid to obtain FeCl 2 solution. The distillation temperature is 95°C, the vacuum degree is 500Pa, and the distillation time is 90min. The water in the spent acid evaporates 84% of the total water to obtain FeCl 2 4H 2 O crystallization and FeCl 2 mother liquor. Get FeCl 2 The content is 98.396%.
[0043] (4) FeCl 2 4H 2 O crystallization an...
Embodiment 2
[0049] The chemical analysis of the waste acid of the sample hot-dip galvanizing is as follows: FeCl 2 : 8%, FeCl 3 : 0.1%, HCl: 0.2%, impurity: 0.3%, and the balance is water.
[0050] Experimental steps:
[0051] (1) Filter the waste acid to remove suspended matter, solid floating matter, etc.
[0052] (2) Add a certain amount of reducing agent (the reducing agent is iron powder) to the waste acid filtered by the plate and frame filter to make Fe 3+ Convert to Fe 2+ . The weight ratio of reducing agent to waste acid is 1:100.
[0053] (3) Put the obtained waste acid into a vacuum distillation furnace and perform low-temperature vacuum evaporation to obtain FeCl 2 solution. The temperature in the vacuum furnace is 60° C., the vacuum degree is 10 Pa, and the evaporation time is 30 minutes. The water in the waste acid evaporates 40% of the total water to obtain FeCl 2 4H 2 O crystallization and FeCl 2 mother liquor. Get FeCl 2 The content is 98.558%.
[0054] (4) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com