A bifunctional anti-tissue factor humanized antibody and its preparation method
A humanized antibody and tissue factor technology, applied in biochemical equipment and methods, chemical equipment and methods, botany equipment and methods, etc., can solve the problems of clinical application of humanized antibodies and reduce parental mouse monoclonal antibodies Immunogenicity, antibody affinity and specificity reduction and other issues, achieve the effect of inhibiting coagulation and tumor cell growth, strong anticoagulant and antitumor activity, and good antigen affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Sequence Analysis and Humanization of TF Murine Antibody
[0028] (1) Sequence analysis of variable region of TF murine antibody
[0029] Using the vector phoA-VH-Linker-VL-His prepared from TF single-chain antibody (CN 103342752 A) as a template, the VH and VL gene sequences were obtained by PCR amplification. Then it was cloned into pUC57 vector to construct pUC57-VH and pUC57-VL vectors. PCR reaction system: 2 µl of 10 µM primers, 30 ng template, Prime STAR R Take 25µl of Max Premix, H 2 O20 µl, the total reaction system is 50 µl. PCR reaction conditions: pre-denaturation at 94°C for 5 min; denaturation at 94°C for 30 s, annealing at 60°C for 30 s, extension at 72°C for 1 min, 30 cycles; extension at 72°C for 10 min; 1% agarose electrophoresis gel to recover VH and VL purposes fragment.
[0030] The primers for cloning VH and VL are as follows:
[0031] VH-F: 5' TCTAGAAAGCTT GCCGCCACCATGGAGATCCAG 3'
[0032] VH-R: 5' GGATCCGAATTC TTATGAGGAGACTGT...
Embodiment 2
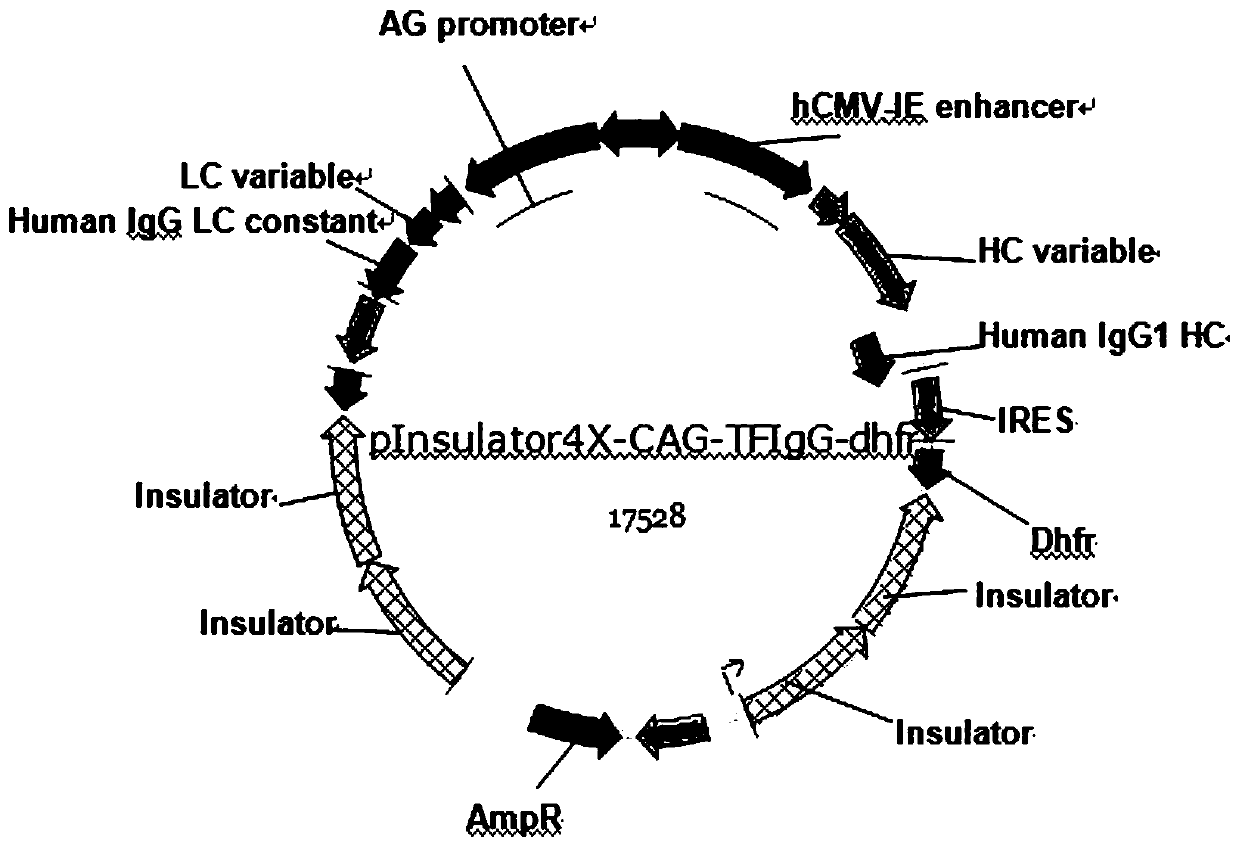
[0079] Example 2: Construction of pinsulator4X-CAG-TFIgG-dhfr expression vector
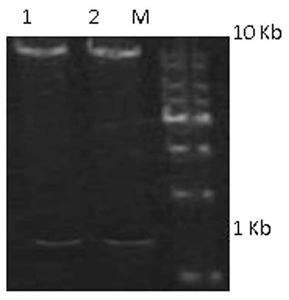
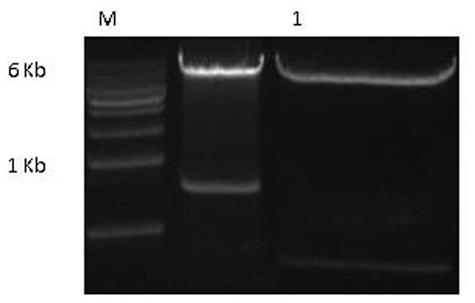
[0080] After the hHC gene sequence was synthesized, Sal I and Asc I double-digested the hHC gene fragment and the pinsulator4X-MSA vector respectively, electrophoresed on a 1% agarose gel, and recovered the insert1 fragment (1425 bp) and the vector1 fragment (11938 bp). The ligation product was formed overnight at 4°C. After the hLC gene sequence was synthesized, N ot I and B The hLC gene and the pCAGGS-IRES-AscI vector were double-digested with ci I, and the insert2 fragment (720 bp) and vector2 fragment (6838 bp) were recovered by electrophoresis gel, and the two were ligated overnight at 4°C to form a ligation product. Transfer the ligation product to a competent state E In .coli DH5α, single colonies were screened with ampicillin. Single colonies were cultured overnight at 37°C, plasmids were extracted from the cells, and identified by enzyme digestion.
[0081] S al I and A sc I doubl...
Embodiment 3
[0086] Embodiment 3: Expression and purification of TF humanized antibody
[0087] 1. Expression of TF humanized antibody
[0088] CHO-dhfr - Cells in 6×10 5 The density was inoculated into 6-well plates for overnight culture. Preparation of transfection complex: add 25 ul Opti-MEM and 4 ul Lipofectamine3000 to tube A, shake at room temperature for 1-2 s, add 25 ul Opti-MEM and 2 ug plasmid to tube B. Mix the above two tubes, let stand for 5 min, and then add to the 6-well culture plate. After 24 hours, cells were trypsinized into culture dishes, and MTX with a final concentration of 50 um was added for pressurized selection of cells. After 15-20 days, single cell clusters were picked and cultured in 6-well plates. When the confluence of the cells reached 90%, they were transferred to T25 culture flasks for growth. Cell density reaches 5 x 10 per ml 5 Transfer the amount of cells into Erlenmeyer flask at 37°C, 5% CO 2 , 130 rpm shaking culture, after 10-15 days of cultu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com