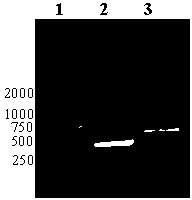
Recombinant phage double expression vector and application
A bacteriophage and double expression technology, applied in the field of recombinant bacteriophage double expression vectors, can solve the problems of unsatisfactory immune effect and the like, and achieve the effects of convenient large-scale production, low cost and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Construction of recombinant phage T7-VP1 129-169
[0036] recombinant phage T7-VP1 129-169 It is formed by inserting the nucleotide sequence encoding the VP1 structural protein (129-169) polypeptide downstream of the p10B gene of T7 Select 415-1b, and the carboxy-terminal of the capsid protein p10B is fused to express the VP1 structural protein of the foot-and-mouth disease virus (129-169) position polypeptide.
[0037] Polypeptide A is formed by adding a flexible Linker amino acid GGGGS to the amino-terminus of the polypeptide at position (129-169) of the VP1 structural protein of foot-and-mouth disease virus (as shown in SEQ ID NO:3). A restriction site EcoRI was added to the 5' end of the nucleotide sequence encoding polypeptide A, and a restriction site Hind III was added to the 3' end to form the DNA sequence shown in SEQ ID NO: 1.
[0038] The DNA sequence shown as SEQ ID NO: 1 was synthesized by a commercial company, and cloned into the multiple clon...
Embodiment 2
[0054] Example 2 Construction of Homologous Recombination Intermediate Plasmid Vector
[0055] Analysis of recombinant phage T7-VP1 129-169 For the genome, the 578th position A on the left side of the genome was selected as the insertion site for the eukaryotic expression cassette. recombinant phage T7-VP1 129-169 The gene fragment at positions 1-578 on the left side of the genome is used as the left homology arm, and the gene segment at positions 2746-2946 on the left side of the genome is the right homology arm. Two pairs of primers were designed for the two homology arms, and XhoI, HindIII, EcoRI, BamHI restriction sites were added at the 5' end of the primers. The left homology arm primers are L1-578UP and L1-578DOWN, and the right homology arm primers are R2746-2946UP and R2746-2946DOWN.
[0056] L1-578UP: 5'-aactcgagTCTCACAGTGTACGGACCTA,
[0057] L1-578DOWN: 5'-AAAAGCTTTCGTGCGACTTATCAGGCTG.
[0058] R2746-2946UP: 5'-AAGAATTCcaGAATTCCAGAAAGAAATTGACCGCGC,
[0059] R...
Embodiment 3
[0076] Example 3 Construction of recombinant T7 phage inserted into eukaryotic expression cassette
[0077] Homologous recombination plasmid pBluescript-L-VP1-R and recombinant phage T7-VP1 129-169 After DNA homologous recombination, the eukaryotic expression cassette composed of CMV eukaryotic promoter, foot-and-mouth disease virus VP1 structural protein gene sequence and SV40 polyA was inserted into the recombinant phage T7-VP1 129-169 After the 578th A on the left side of the genome, the recombinant phage T7-VP1 was replaced 129-169 Fragment at position 579-2745 in the genome to obtain recombinant phage T7-VP1 129-169 -VP1.
[0078] The specific method of homologous recombination is as follows: import the intermediate vector pBluescript-L-VP1-R into E. coli In BL21, BL21-pBluescript-L-VP1-R was obtained. Streak inoculate BL21-pBluescript-L-VP1-R on the LB solid medium plane containing ampicillin resistance, culture overnight at 37°C; pick a single colony from the plat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com