3-coumarin formic acid compound and application thereof as antibacterial agent for preparing phytopathogens
A plant pathogenic bacteria, coumarin technology, applied in the direction of plant growth regulators, applications, fungicides, etc., can solve serious diseases and other problems, achieve the effect of small molecular weight, good activity, and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The synthetic route for preparing 3-coumarin carboxylic acid compounds established in the present invention is as follows:
[0034] (1) Synthesis of 3-coumarin ethyl formate compounds
[0035] Using substituted salicylaldehyde as a raw material, under the catalysis of piperidine, it was refluxed with diethyl malonate in absolute ethanol for 2 to 5 hours, and then Knoevnagal condensation reaction occurred, and 14 ethyl 3-coumarin formate were prepared. Esters (A1-A14).
[0036]
[0037] (2) Synthesis of 3-coumarin formic acid compounds
[0038] Ethyl 3-coumarin carboxylate compounds are hydrolyzed under alkaline conditions and then acidified to obtain the corresponding 3-coumarin carboxylate compounds (B1-B14).
[0039]
[0040] The specific operation steps for the synthesis of different types of 3-coumarin formic acid compounds are as follows:
[0041] 1. Compound A1——Synthesis of 3-coumarin ethyl carboxylate
[0042] Into a dry round bottom flask were added salicylaldehyde (4.9g,...
example 1
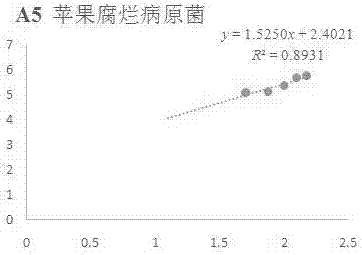
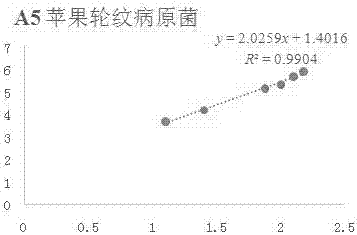
[0074] Example 1 In vitro antibacterial activity of compound A5 against apple rot pathogenic fungi
[0075] Accurately weigh 5 mg of compound A5 in a 20 mL vial, inject 0.5 mL of DMSO and 9.5 mL of sterile water into it, prepare the test sample into a 5% DMSO solution; then add it to the 90 mL of PDA culture medium that has been melted after sterilization, Mix well, prepare 100 mL of culture medium containing the sample to be tested, pour it into a sterile petri dish, and make a flat culture medium with medicine. The same amount of solvent, 5% DMSO, was used as a blank control, and the commercially available antibacterial drug Kresoxim-methyl was used as a positive control.
[0076] Use a punch with a diameter of 5mm to take out the vigorously growing apple rot pathogenic bacteria cake on the edge of the colony, and transfer it to the above-mentioned flat medium with an inoculating needle, with the hyphae facing down, stick to the medium, 3 pieces per dish , Placed in a triangle i...
example 2
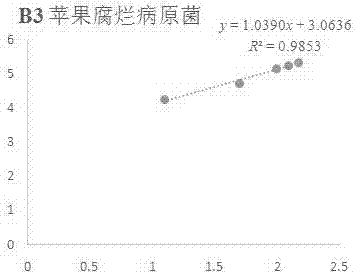
[0080] Example 2 In vitro antibacterial activity of compound B3 against corn curvularia pathogen
[0081] Accurately weigh 5 mg of compound B3 in a 20 mL vial, inject 0.5 mL of DMSO and 9.5 mL of sterile water into it, prepare the test sample into a 5% DMSO solution; then add it to the 90 mL of PDA culture medium that has been melted after sterilization, Mix well, prepare 100 mL of culture medium containing the sample to be tested, pour it into a sterile petri dish, and make a flat culture medium with medicine. The same amount of solvent, 5% DMSO, was used as a blank control, and the commercially available antibacterial drug Kresoxim-methyl was used as a positive control.
[0082] Use a punch with a diameter of 5mm to take out the vigorously growing corn curvularia pathogen cake on the edge of the colony, transfer it to the above-mentioned flat medium with an inoculation needle, and stick the hyphae side down on the medium, 3 per dish The block is placed in the center in a triangl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com