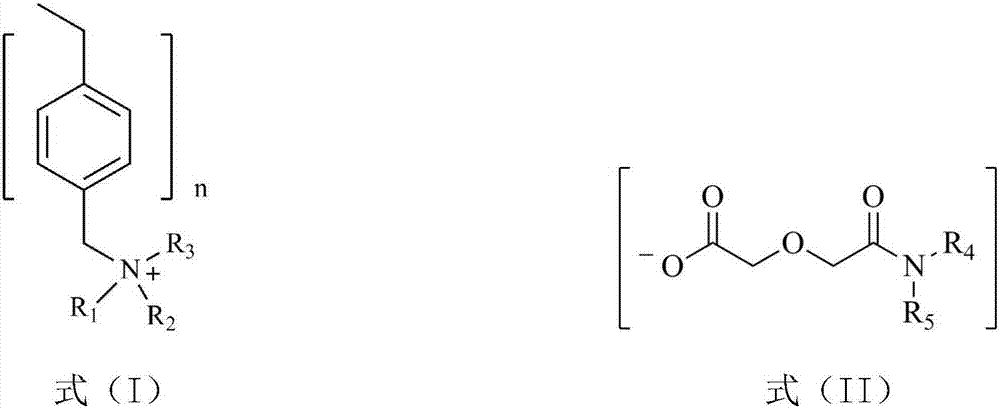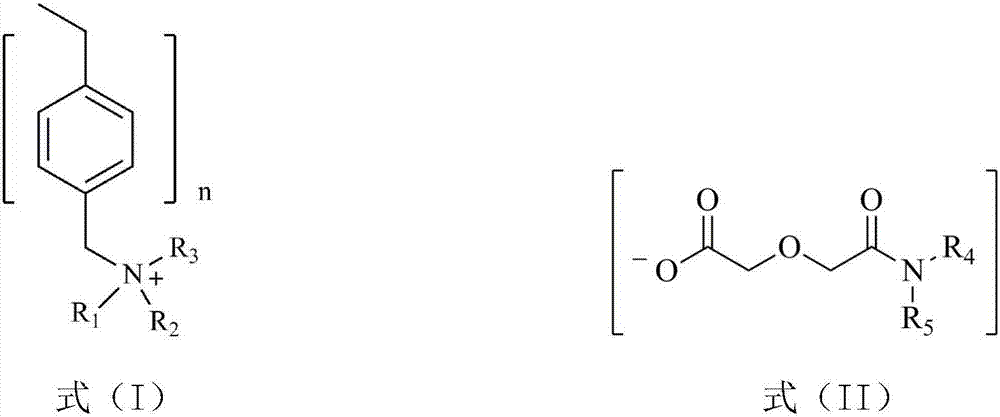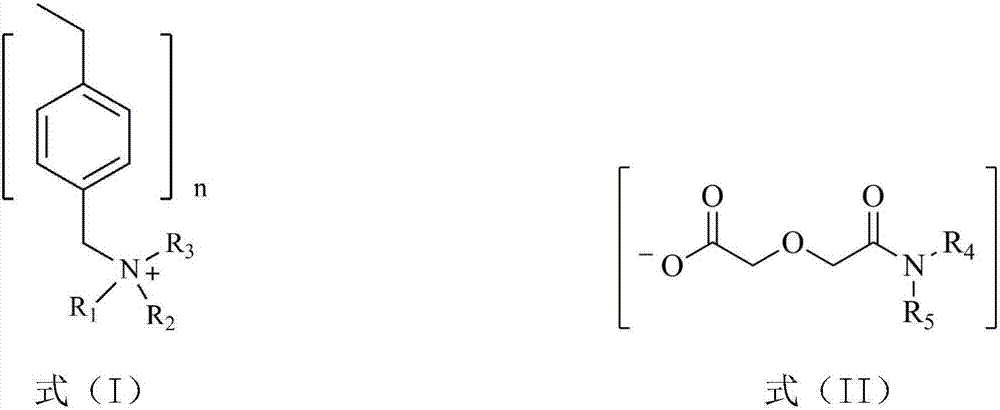Solid-supported ionic liquid and preparation method thereof
An ionic liquid and cation technology, applied in the field of immobilized ionic liquid and its preparation, can solve the problems of low impurity removal efficiency and low recovery rate of rare earth ions, and achieve the effects of environmental protection, superior impurity removal performance and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The above-mentioned immobilized ionic liquid preparation method comprises the following steps:
[0028] (1) prepare dialkyl diglycol amic acid;
[0029] Dissolve dialkylamine and diglycolic anhydride in a low-polarity organic solvent, stir and react at room temperature for 48 hours, wash the resulting solution with dilute hydrochloric acid solution to remove residual diisooctylamine, and rotary evaporate to remove ethyl acetate and a small amount of water to obtain dialkyl diglycol amic acid. Low polar organic solvents include ethyl acetate, tetrahydrofuran, toluene, hexane or kerosene; ethyl acetate is preferred. To ensure complete reaction of diglycolic anhydride, the present invention sets the molar ratio of dialkylamine to diglycolic anhydride to be 1.0-1.5:1.
[0030] Dialkylamines are preferred: diisooctylamine, di-n-octylamine, di-n-hexylamine or di-n-decylamine.
[0031] The dialkyl diglycol amic acid prepared by selecting the above raw materials is: dioctyl ...
Embodiment 1
[0040] (1) Diisooctylamine and diglycolic anhydride with a molar ratio of 1.1:1 were dissolved in ethyl acetate, stirred and reacted at room temperature for 48 hours, and the resulting solution was washed with dilute hydrochloric acid solution to remove residual diisooctylamine, Ethyl acetate and a small amount of water were removed by rotary evaporation to obtain di(2-ethylhexyl) diglycol amic acid;
[0041] (2) Put chlorine-type strong basic polystyrene resin in a chromatographic column, and use 2wt% sodium hydroxide solution to flow through the resin layer to carry out anion exchange. After the alkali washing is completed, rinse with deionized water until the pH value is neutral to obtain a strongly basic polystyrene resin in the form of hydroxide.
[0042](3) Swell 20.0g of hydroxide-type strongly basic polystyrene resin in N,N-dimethylformamide for 1h to make a suspension, and then 29.1g of bis(2-ethylhexyl) diglycolamide Dissolve the acid in N,N-dimethylformamide, add i...
Embodiment 2
[0045] (1) Dissolve di-n-octylamine and diglycolic anhydride with a molar ratio of 1.3:1 in ethyl acetate, stir and react at room temperature for 48 hours, and use dilute hydrochloric acid solution to wash the resulting solution to remove residual di-n-octylamine, Ethyl acetate and a small amount of water are removed by rotary evaporation to obtain dioctyl diglycol amic acid;
[0046] (2) Put chlorine-type strong basic polystyrene resin in a chromatographic column, and use 2wt% sodium hydroxide solution to flow through the resin layer to carry out anion exchange. After the alkali washing is completed, rinse with deionized water until the pH value is neutral to obtain a strongly basic polystyrene resin in the form of hydroxide.
[0047] (3) Swell 15.0g of hydroxide-type strongly basic polystyrene resin in N,N-dimethylformamide for 1h to make a suspension, then dissolve 19.3g of dioctyl diglycol amic acid in N, Add N-dimethylformamide to the suspension of the above-mentioned hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com