Synthesis method of ciclopirox olamine
A synthesis method and technology of ciclopirox amine, which is applied in the field of synthesis of ciclopirox olamine, can solve problems such as the lack of a synthesis method of ciclopirox olamine, and achieve the effects of high yield, good stability, and low operating cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
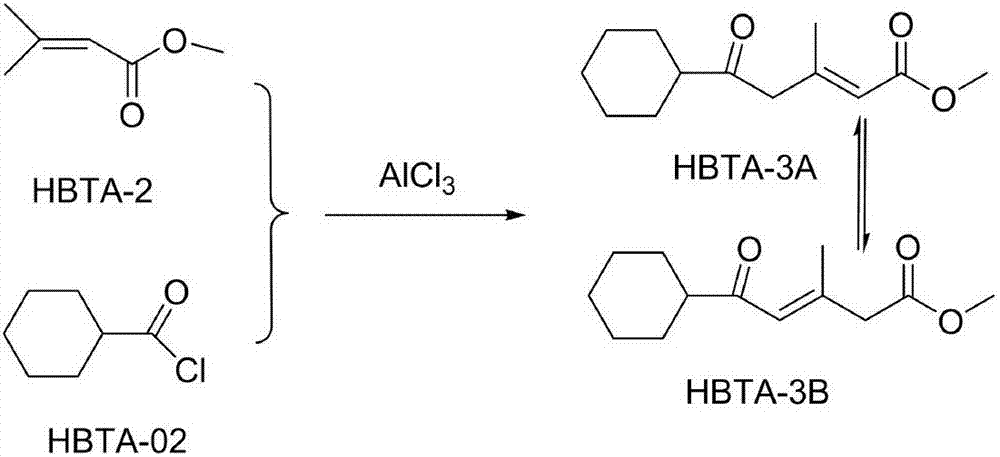
[0032] A kind of synthetic method of ciclopirox olamine of the present invention, comprises the steps:
[0033]
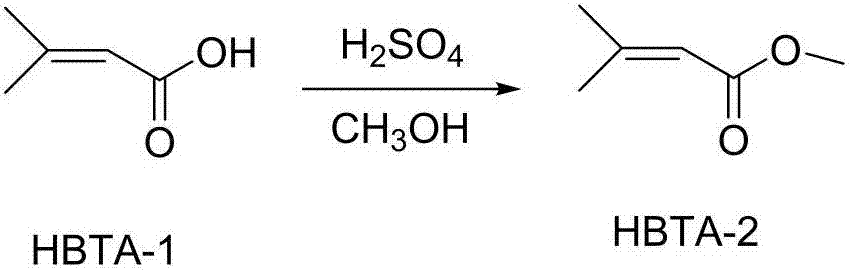
[0034] (1) Preparation of methyl dimethacrylate: add dimethacrylic acid, methanol and concentrated sulfuric acid to the reaction flask, heat and reflux for 5 hours, distill methanol, pour the residue into ice water, separate the ester layer, water layer was extracted with dichloromethane, the ester layer was combined with dichloromethane, washed with sodium bicarbonate solution, and then washed with water until neutral, dried over sodium sulfate, distilled out dichloromethane, and then distilled under reduced pressure to collect 37 ° C / 2400Pa Distillate, get product; The volume ratio of described methanol, concentrated sulfuric acid, ice water is 35:1.4:30; The mass volume ratio of described dimethacrylic acid and methanol is 0.286g / ml.
[0035]
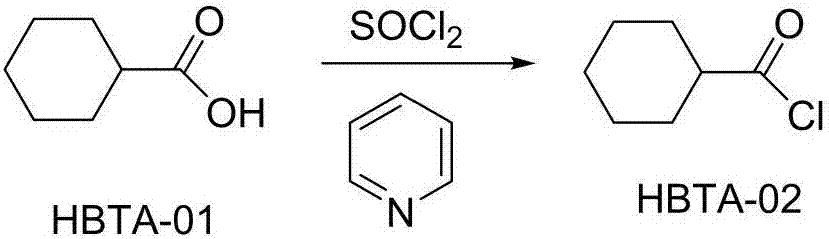
[0036] (2) Preparation of cyclohexanecarbonyl chloride:
[0037] Add cyclohexane carboxylic acid and catalytic...
Embodiment 2
[0051] The difference between embodiment 2 and embodiment 1 is: a kind of synthetic method of ciclopirox amine of the present invention, comprises the steps: in step (1), the dimethacrylic acid of 10g, 35ml methyl alcohol and 1.4ml vitriol oil add In the reaction bottle, heat to reflux for 5 hours, distill off methanol, pour the residue into 30ml of ice water, separate the ester layer, extract the water layer with dichloromethane, combine the ester layer with dichloromethane, and wash with sodium bicarbonate solution , then washed with water until neutral, dried over sodium sulfate, distilled dichloromethane, and then distilled under reduced pressure to collect fractions at 40°C / 2400Pa to obtain 8.9g of the product with a yield of 78%.
[0052] In step (2), 20 g of cyclohexanecarboxylic acid and pyridine of catalytic amount are added in the reaction flask, and slowly add thionyl chloride dropwise, and the control time is dropped in 30 minutes, and continue to stir and heat to r...
Embodiment 3
[0056] The difference between embodiment 3 and embodiment 1 is:
[0057] A kind of synthetic method of ciclopirox olamine of the present invention comprises the following steps: (1) preparing methyl dimethacrylate: adding dimethacrylic acid, methanol and concentrated sulfuric acid in the reaction flask, heating to reflux for 5 hours, distilling Methanol was removed, the residue was poured into ice water, the ester layer was separated, the water layer was extracted with dichloromethane, the ester layer was combined with dichloromethane, washed with sodium bicarbonate solution, and then washed with water until neutral, dried over sodium sulfate, Distill out dichloromethane, and then distill under reduced pressure, collect the cut of 39 ℃ / 2400Pa, obtain product; The volume ratio of described methanol, concentrated sulfuric acid, ice water is 35:1.4:30; The dimethacrylic acid and methanol The mass volume ratio is 0.286g / ml.
[0058] (2) Preparation of cyclohexanecarbonyl chloride...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com