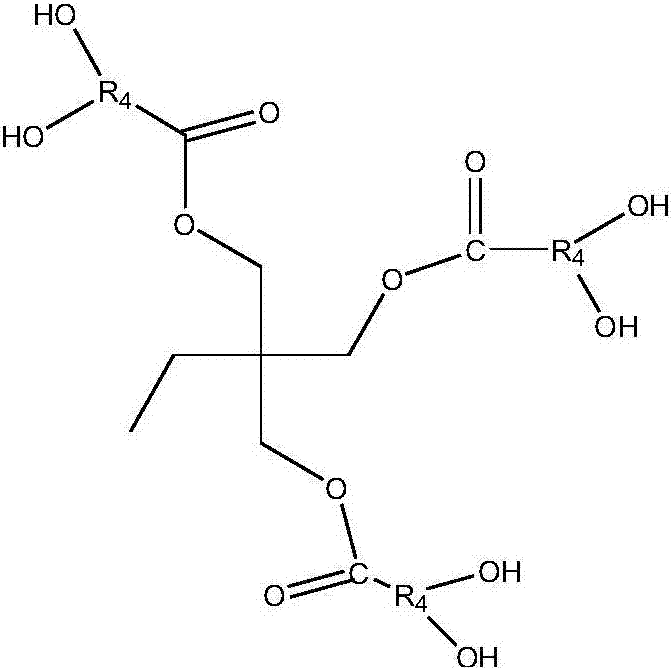
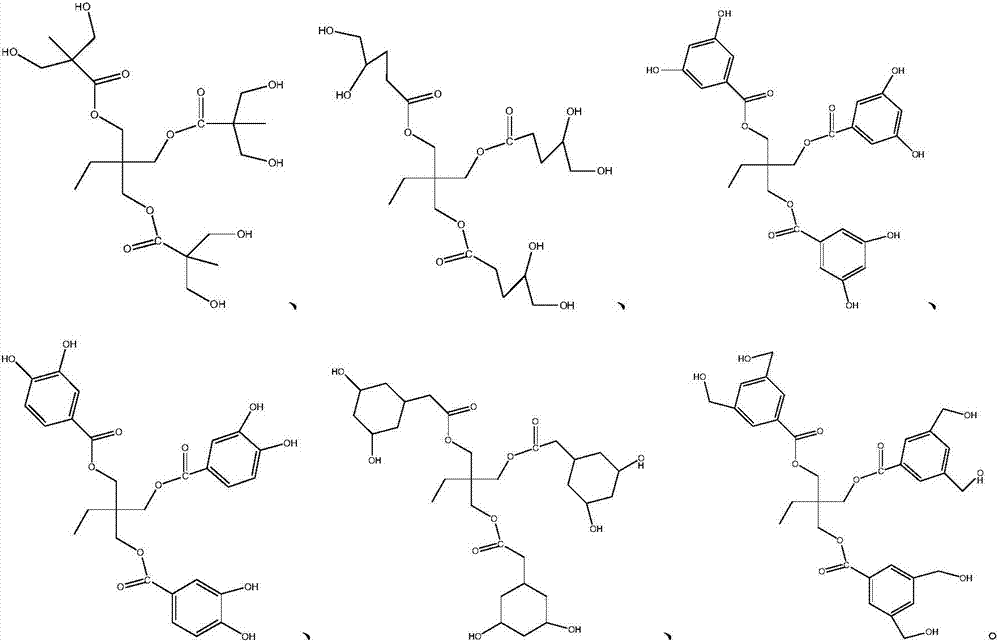
Preparation method of isocyanates
A technology of isocyanate and diisocyanate, which is applied in the preparation of isocyanate derivatives, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of reduced production efficiency, low concentration of salt formation, and reduced economic efficiency, so as to avoid yellowing change, the effect of low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Add 50 g (0.1 mol, about 0.5 mol of hydroxyl groups) of hydroxyl-terminated hyperbranched polyester HyPer H101 into 75 g of solvent butyl acetate to make the solid content 40 wt%. Add 87gTDI (0.5mol) and catalyzer dibutyltin dilaurate 0.06g in the four-neck flask equipped with stirrer, thermometer and condensing tube, under constant stirring at constant speed, slowly add the prepared HyPer H101 butyl acetate solution dropwise, drop At the end of the addition, the temperature was slowly raised to 60°C. When the system-NCO content was 50% of the initial NCO content, 300g of butyl acetate was added to reduce the viscosity of the system, and the above mixed solution was added to 54g of 4-(aminomethyl)pyridine (0.5mol ), the end-capping reaction was carried out at 80°C, and the NCO content of the system was 1wt%. The reaction was completed, and the polyester hyperdispersant 1 with a solid content of 34wt% was obtained, and its molecular weight was 1911.
Embodiment 2
[0072]Add 60 g (0.1 mol, about 0.5 mol of hydroxyl group) of hydroxyl-terminated hyperbranched polyester HyPer H201 into 115 g of solvent butyl acetate to make the solid content 35 wt%. Add 87g TDI (0.5mol) and catalyst stannous octoate 0.08g in the four-neck flask equipped with stirrer, thermometer and condenser tube, under constant stirring at a constant speed, slowly add the HyPerH201 butyl acetate solution prepared dropwise at a constant temperature, and the dropwise addition ends slowly Heat up to 70°C, when the system-NCO content is 52% of the initial NCO content, add 300g butyl acetate to reduce the viscosity of the system, and add 80g 8-amino-2-methylquinoline (0.5mol) to the above mixed solution During the end-capping reaction at 70°C, the NCO content of the system was 0.3wt%, and the reaction was completed to obtain a polyester hyperdispersant 2 with a solid content of about 35wt%, and its molecular weight was 2261.
Embodiment 3
[0074] Add 83 g (0.09 mol, about 0.45 mol of hydroxyl group) of hydroxyl-terminated hyperbranched polyester HyPer H301 into 140 g of solvent butyl acetate to make the solid content 37%. Add 125gMDI (0.5mol) and 0.06g of catalyst stannous octoate into a four-necked flask equipped with a stirrer, a thermometer and a condenser tube, and slowly add the configured HyPerH301 butyl acetate solution dropwise at a constant temperature under constant stirring, and the dropwise addition ends slowly. Heat up to 80°C, when the system-NCO content is 51% of the initial NCO content, add 300g butyl acetate to reduce the viscosity of the system, and add the above mixed solution to 60g 4-amino-2-picoline (0.55mol) , Carry out capping reaction at 60°C, the NCO content of the system is 0.15wt%, the reaction is completed, and the polyester hyperdispersant 3 with a solid content of about 38% is obtained, and its molecular weight is 2710.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com