Vero E6 cell strain adapting to full-suspension culture and application thereof
A technology of veroe6-s and cell lines, applied in animal cells, vertebrate cells, urinary tract/kidney cells, etc., can solve the problems of inability to achieve optimal growth in cell culture, high labor intensity, and difficulty in cell domestication, and achieve Good industrial application prospects, high degree of automation, and the effect of meeting the requirements of large-scale industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Adapt to the cultivation of the Vero E6-S cell strain of full suspension culture
[0030] Vero E6-S is preserved in the China Center for Type Culture Collection with the preservation number CCTCC C2017101.
[0031] ①Take a well-growing Vero E6-S cell line, add it to a shake flask filled with nutrient solution, culture it at 36-37°C for 72-96 hours, and subculture and enlarge it according to the ratio of 1:3-1:4;
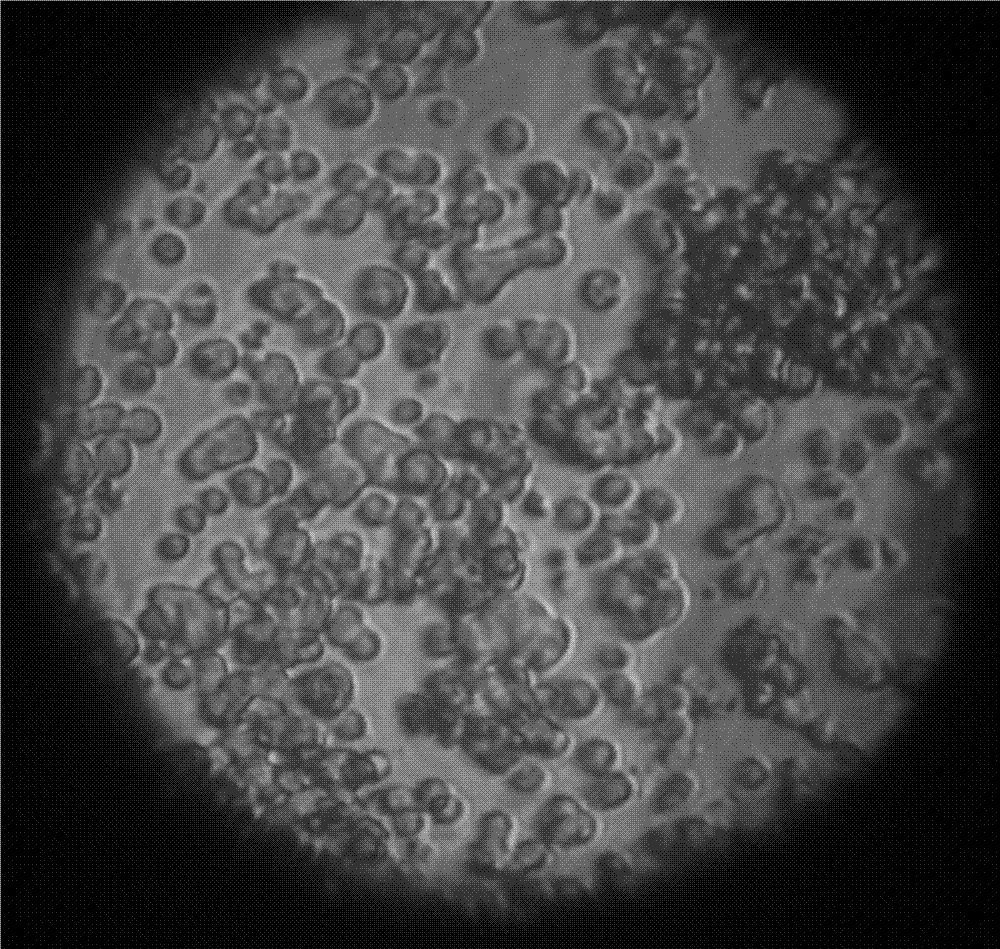
[0032] ② Dilute the Vero E6-S cell line amplified in step ① to (6-10)×10 5 Cells / ml density, inoculated into bioreactor, culture temperature is 36-37℃, pH value is 6.8-7.2, dissolved oxygen is 40%-60%, Vero E6-S cell suspension growth curve is as follows: figure 1 As shown, suspension growth pictures see figure 2 ;
[0033] ③Determination of cell density in three batches of bioreactors: three batches of Vero E6-S cells were continuously cultured in bioreactors, the culture conditions were the same as in step ②, samples were taken at the 72nd ...
Embodiment 2
[0035] Embodiment 2: utilize Vero E6-S suspension culture cell to cultivate the method for porcine epidemic diarrhea virus
[0036] Take well-grown suspension Vero E6-S cells, press 6.5×10 5 Cells / ml density, inoculated into bioreactor, cell culture conditions: reactor working volume 5.0 liters, cell culture temperature 37 ℃, pH value 7.0, dissolved oxygen 50%, culture 72 hours, cell density 44.7 ×10 5 Individuals / ml, replace with the virus culture maintenance medium that contains 1.0% newborn bovine serum, add trypsin according to the amount of 10ug / ml, inoculate porcine epidemic diarrhea virus according to 10% of virus culture maintenance medium volume at the same time, virus culture The conditions are: temperature 36.5°C, pH value 6.9, dissolved oxygen 45%, culture for 48 hours to harvest the virus liquid, the virus titer is determined according to the standard of "Regulations of Veterinary Biological Products of the People's Republic of China", and the harvested virus con...
Embodiment 3
[0037] Embodiment 3 Utilize the method of Vero E6-S suspension culture cell culture porcine pseudorabies virus
[0038] Take well-grown suspended Vero E6-S cells, press 7.4×10 5 Cells / ml density, inoculated into bioreactor, cell culture conditions: reactor working volume 5.0 liters, cell culture temperature 36.5 ℃, pH value 6.8, dissolved oxygen 50%, culture 88 hours, cell density 47.1 ×10 5 Each / ml, replace with the virus culture maintenance medium containing 2.0% newborn bovine serum, and inoculate porcine pseudorabies virus according to 5% of the volume of the virus culture maintenance medium at the same time, the virus culture conditions are: temperature 36.5 ℃, pH value 7.0 , the dissolved oxygen was 55%, and the virus liquid was harvested after 36 hours of cultivation. The virus titer was judged according to the "Regulations of Veterinary Biological Products of the People's Republic of China", and the harvested virus content was 8.5 TCID 50 / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com