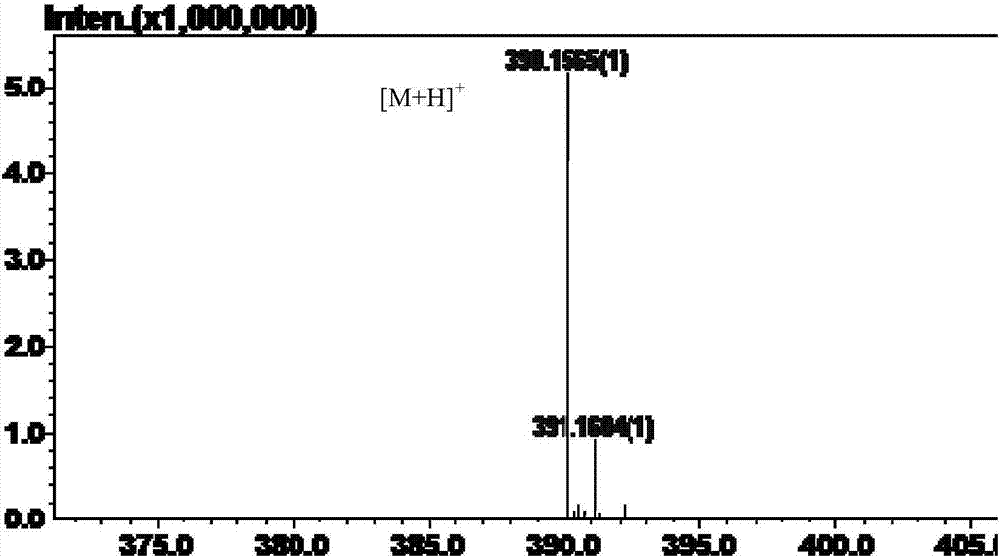
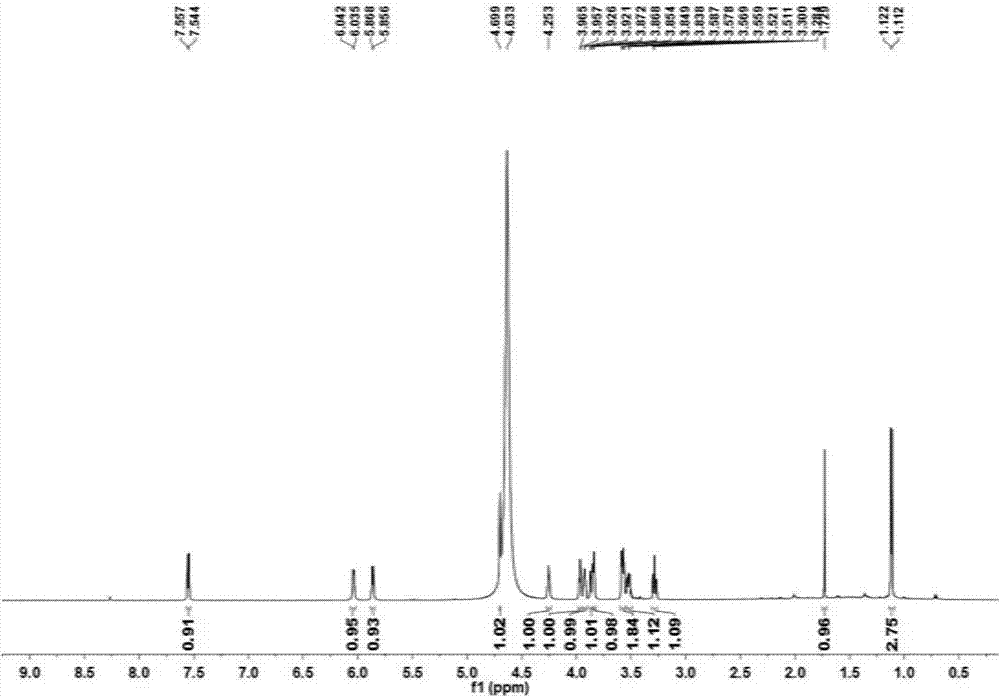
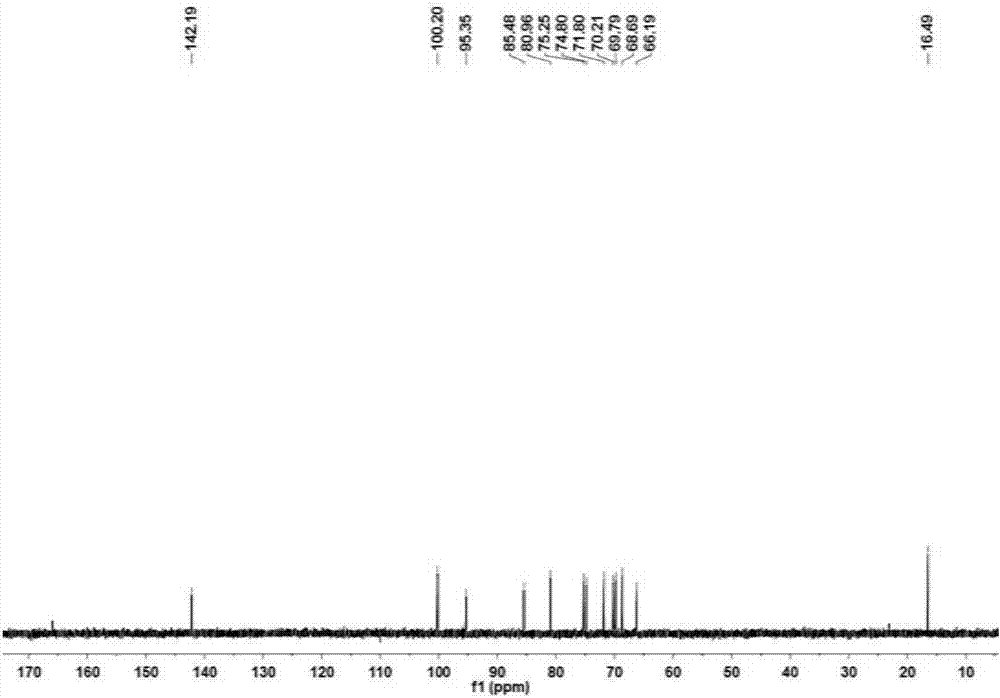
Application of alpha-L-rhamnosidase in preparing cytarabine derivative
A technology of rhamnosidase and cytarabine, which is applied in the field of synthesizing cytarabine rhamnoside by α-L-rhamnosidase, and can solve the unrelated reports on the glycosylation modification of cytarabine, etc. problem, to achieve the effect of increasing targeting and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation method of cytarabine rhamnoside, the steps are as follows:
[0041] 1. Preparation of α-L-rhamnosidase
[0042] Artificially synthesize the α-L-rhamnosidase sequence with GenBank accession number JN704640 (encoded protein GenBank accession number is AFA41506.1, nucleotide sequence such as SEQ ID NO.2), connect it to the pPIC9K plasmid, and transform PichiapastorisGS115 . The α-L-rhamnosidase was prepared according to the instructions of the Pichia pastoris expression operation manual provided by Invitrogen, and the protein content of the enzyme was determined by the Coomassie brilliant blue method. After detection, the amino acid sequence was shown in SEQ ID NO.1.
[0043] 2. α-L-rhamnosidase catalyzes the synthesis of cytarabine rhamnoside
[0044] (1) Use sodium phosphate buffer to prepare rhamnose concentration of 1.1M, cytarabine concentration of 0.5M, amino acid sequence as shown in SEQ ID NO.1 α-L-rhamnosidase addition amount is 10 μg / mL of rea...
Embodiment 2
[0056] A method for enzymatically preparing cytarabine rhamnoside, comprising the steps of:
[0057] (1) Prepare rhamnose concentration of 0.6M and cytarabine concentration of 0.4M with sodium phosphate buffer solution, the amino acid sequence is as follows
[0058] A reaction system in which the amount of α-L-rhamnosidase shown in SEQ ID NO.1 is 8 μg / mL;
[0059] The buffer in the step (1) is a sodium phosphate buffer with a concentration of 10mM and pH6;
[0060] (2) React the reaction system prepared in step (1) in a water bath at 55°C for 30 hours, boil at 100°C for 5 minutes to terminate the reaction,
[0061] Centrifuge at 12,000 rpm for 30 minutes, and take the supernatant;
[0062] (3) The supernatant obtained in step (2) is separated with a preparative thin-layer chromatography plate, and the products with the same migration distance are combined,
[0063] After freeze-drying, it is made into powder, which is cytarabine rhamnoside.
[0064] Thin layer chromatograp...
Embodiment 3
[0069] An enzyme-prepared cytarabine rhamnoside inhibits tumor cells, comprising the steps of:
[0070] (1) Breast cancer MDA-MB-231 cells were cultured to the logarithmic phase, digested with 0.25% trypsin and collected cells, then adjusted the cell concentration to 3×10 4 cells / mL, inoculated in a 96-well plate at 100 μL / well, set 3 parallel experiments for each group, and set corresponding blank group and control group;
[0071] (2) Place the 96-well plate of cells inoculated in step (1) in a 5% CO2, 37°C cell incubator for 24 hours; concentration of cytarabine rhamnoside or anti-tumor drug original drug, the final concentration is 10 μmol / L-1mmol / L, and the control group is supplemented with PBS phosphate buffered saline;
[0072] The PBS phosphate buffered saline solution is 0.01M sodium dihydrogen phosphate, disodium hydrogen phosphate mixed component buffer, pH is 7.4;
[0073] (3) Set the same conditions as the step (2), add the α-L-rhamnosidase derived from Alternar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com