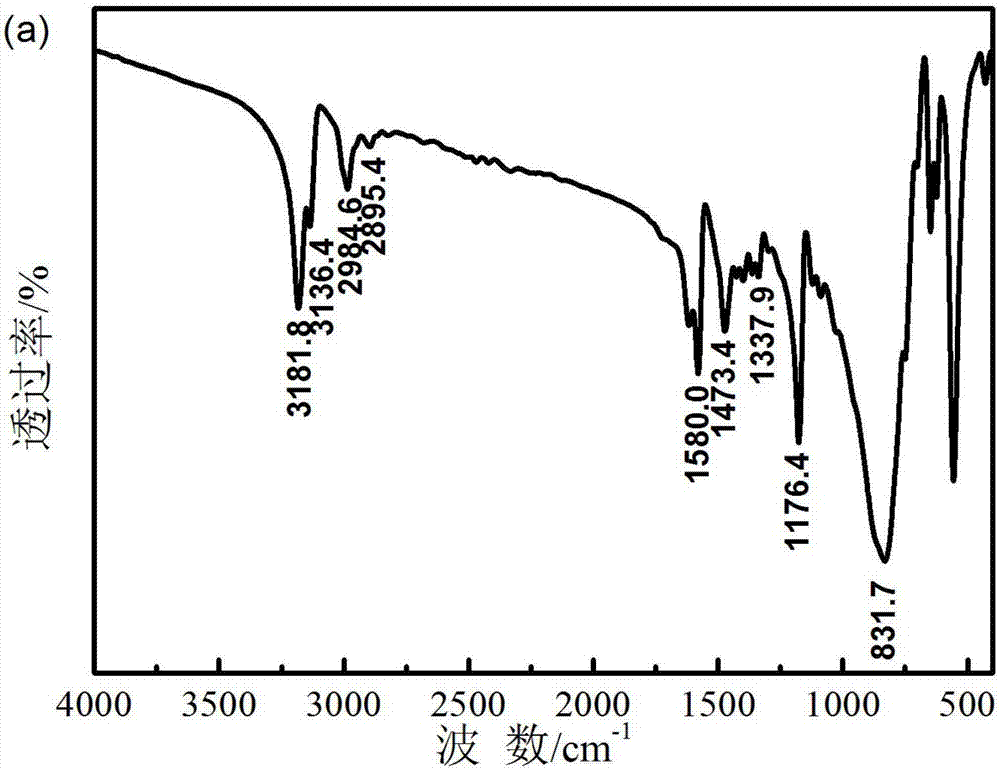
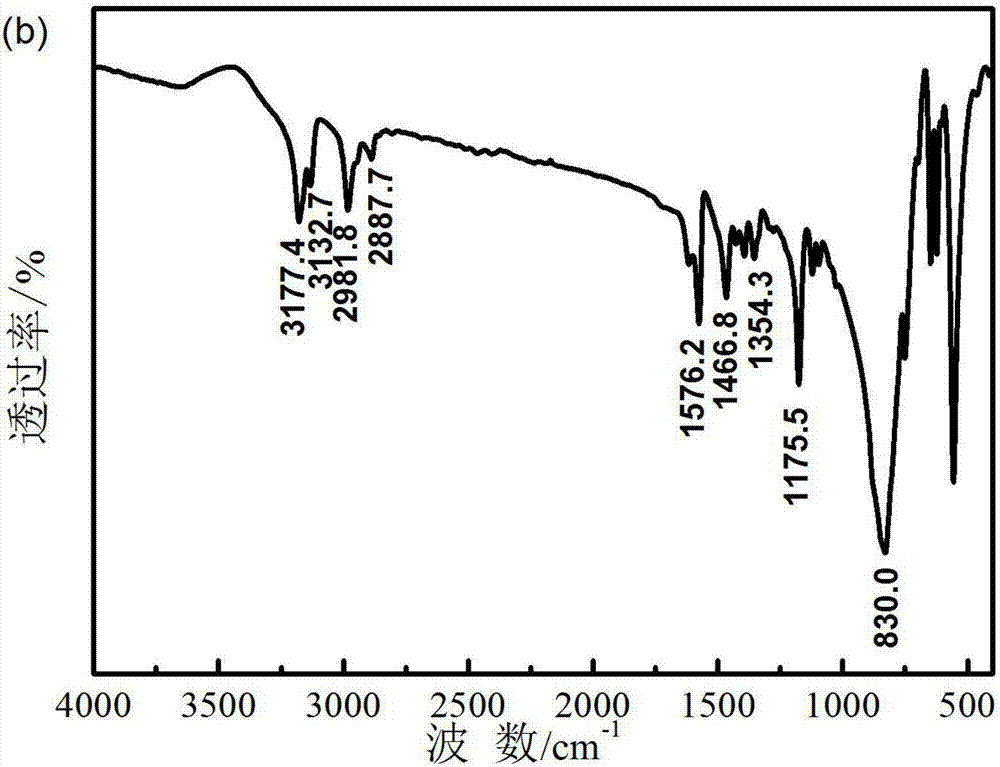
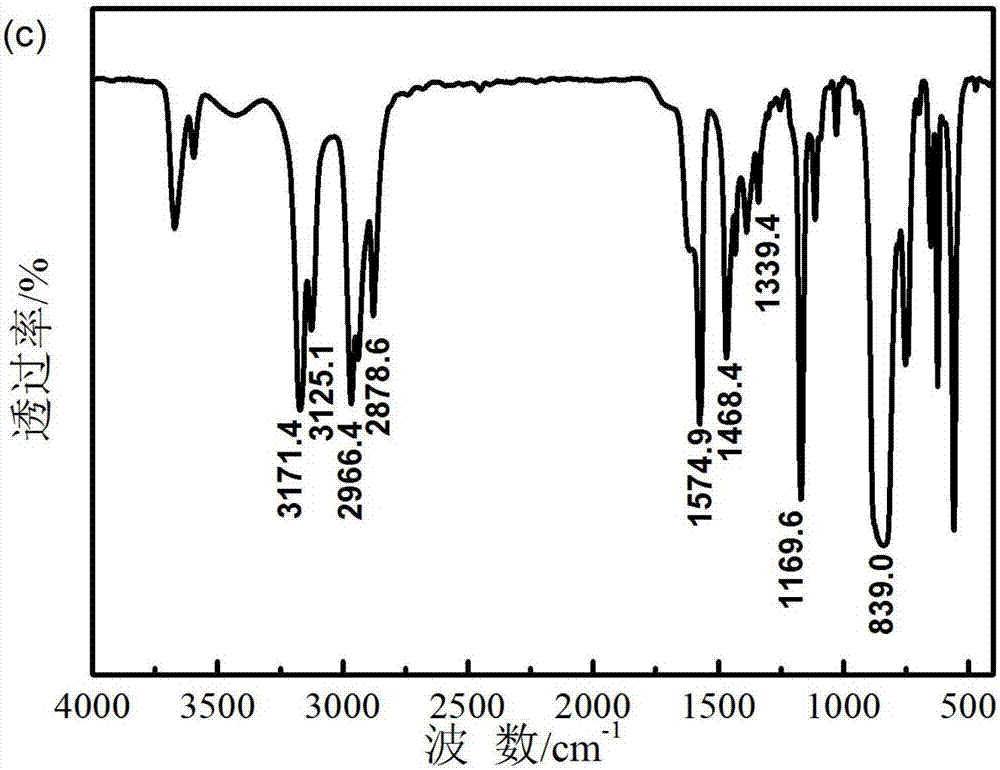
Preparation method of ionic liquid electrolyte system for electrochemical oxygen sensor
An oxygen sensor and ionic liquid technology, applied in the direction of material electrochemical variables, etc., can solve the problems of easy safety problems, small response current, low conductivity, etc., achieve short cycle of shape control, increase response current, and improve conductivity rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Step 1: Weigh 0.05mol 1-methylimidazole and 0.055mol potassium hexafluorophosphate and mix them with 0.055mol ethyl bromide, 0.055mol propane bromide and 0.055mol butyl bromide respectively according to the ratio of 1:1.1:1.1, and use the one-pot method Heat and reflux in a water bath at 80°C for 3 hours, wash with ultra-pure water for 5 times after cooling, check the supernatant with silver nitrate solution for no light yellow precipitate, and obtain 1-ethyl-3-methylimidazole hexafluorophosphate (EMIMPF 6 ), 1-propyl-3-methylimidazolium hexafluorophosphate (PMIMPF 6 ) and 1-butyl-3-methylimidazolium hexafluorophosphate (BMIMPF 6 );
[0031] Step 2: Select the most commonly used BMIMPF 6 0.05mol is mixed with 1-vinylimidazole (VIM) according to the mass ratio of 0%, 20%, 40%, 60%, 80% and 100%, and ultrasonically for 1h to ensure that the mixture is uniform, and the cyclic voltammetry curve test is carried out by finding The highest response current determines the ...
Embodiment 2
[0048] The difference between this embodiment and the first embodiment is that the said alkenylimidazole is 1-allyl-3-vinylimidazole. On this basis, we mixed polyethylene glycol into the final electrolyte, and tested the cyclic voltammetry curves of different scan rates and different oxygen concentrations on the final electrolyte.
[0049] Technical Note: Polyethylene glycol is widely used and researched in organic chemistry. It is usually used as a phase transfer catalyst because its linear polymer chain can form a shape similar to a crown ether. Due to its unique solubility, it is suitable for organic compounds. Separation and purification. In addition, polyethylene glycol, especially polyethylene glycol with a large molecular weight, can also be used as a supporting agent and the like. However, there is no report on adjusting the liquidity of the system. In view of the advantages of low toxicity, harmless to the environment, low melting point, high boiling point, thermal ...
Embodiment 3
[0051] The difference between the previous stage of this embodiment and the second embodiment is that the inorganic catalyst is foamed carbon loaded with nickel. On this basis, lithium bistrifluoromethanesulfonimide (LiTFSI) was added to the blend system, which can not only improve the conductivity but also increase the gas solubility. The final electrolyte was tested by cyclic voltammetry curves with different scan rates and different oxygen concentrations.
[0052] Technical note: LiTFSI is usually used as lithium salt of organic electrolyte for lithium batteries, which has high electrochemical stability and conductivity. In addition, it can be used as a Lewis acid catalyst for organic catalytic cracking, hydrocracking, catalytic reforming, isomerization, olefin hydration, toluene disproportionation, alcohol dehydration and acylation reactions. But there is no report about it as a sensor electrolyte additive. In addition, through Henry's law and gravity analysis microbalan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com