Method for extracting vanadium and chromium from mixed solution containing vanadium and chromium
A mixed solution and mixed solution technology, applied in the field of extracting vanadium and chromium, can solve the problems of long process flow, complicated operation and emulsification, and achieve the effect of simple equipment requirements, short process flow and efficient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
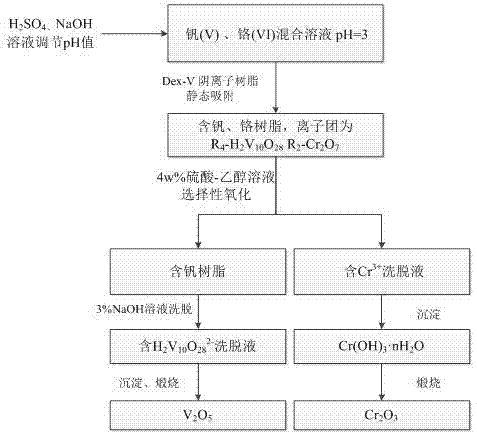
[0030] A 150mL vanadium-chromium mixed solution containing pentavalent vanadium (100mg / L) and hexavalent chromium (100mg / L) was adjusted to pH 8.0 with sodium hydroxide solution or sulfuric acid. The Dex-V macroporous weakly basic anion exchange resin was added to a magnetic heating stirrer to perform a static adsorption experiment at 25°C. The adsorption time was 40 minutes.
[0031] After testing, the adsorption rates of vanadium (V) and chromium (VI) are 93.76% and 99.50%. The effect of pH on the adsorption rate of V(V) and Cr(VI) is as follows: figure 2 Shown.
[0032] Experiments show that under the optimal adsorption temperature and contact time, the adsorption capacity of Dex-V macroporous weakly basic anion exchange resin for vanadium (V) and chromium (VI) has decreased, and the adsorption rate of vanadium has decreased by up to 5%.
Embodiment 2
[0034] A 150mL vanadium-chromium mixed solution containing pentavalent vanadium (100mg / L) and hexavalent chromium (100mg / L) was adjusted to pH 3.0 with sodium hydroxide solution or sulfuric acid. The Dex-V macroporous weakly basic anion exchange resin was added to a magnetic heating stirrer to perform a static adsorption experiment at 25°C. The adsorption time was 40 minutes.
[0035] Use oxalic acid, hydrochloric acid, sulfuric acid, ascorbic acid and 4% sulfuric acid-ethanol solution to elute the vanadium and chromium resins and compare the desorption rates.
[0036] After testing, the desorption rates of oxalic acid on vanadium (V) and chromium (VI) are 0.38% and 0.46%, respectively; the desorption rates of hydrochloric acid on vanadium (V) and chromium (VI) are 51.05% and 1.38%, respectively; The desorption rates of (V) and chromium (VI) are 70.32% and 1.86%, respectively; the desorption rates of ascorbic acid on vanadium (V) and chromium (VI) are 98.53% and 99.25%, respectivel...
Embodiment 3
[0038] A 150 mL vanadium-chromium mixed solution containing pentavalent vanadium (100mg / L) and hexavalent chromium (100mg / L) was adjusted to pH 3.0 with sodium hydroxide solution or sulfuric acid. The Dex-V macroporous weakly basic anion exchange resin was added to a magnetic heating stirrer for static adsorption experiments at 25°C, and the adsorption time was 40 minutes. After testing, the adsorption rate of vanadium (V) and chromium (VI) can reach 98.83% and 99.87%. The vanadium-containing resin and chromium-containing resin are eluted with 4% sulfuric acid-ethanol solution to reach equilibrium, and the vanadium-containing resin and chromium-containing desorption solution are obtained. The desorption rates of the 4% sulfuric acid-ethanol mixture solution on vanadium (V) and chromium (VI) are respectively tested. It is 2.10% and 96.70%. At this time, vanadium is adsorbed on the resin in the form of anion groups. After the chromate ion groups come into contact with the desorp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com