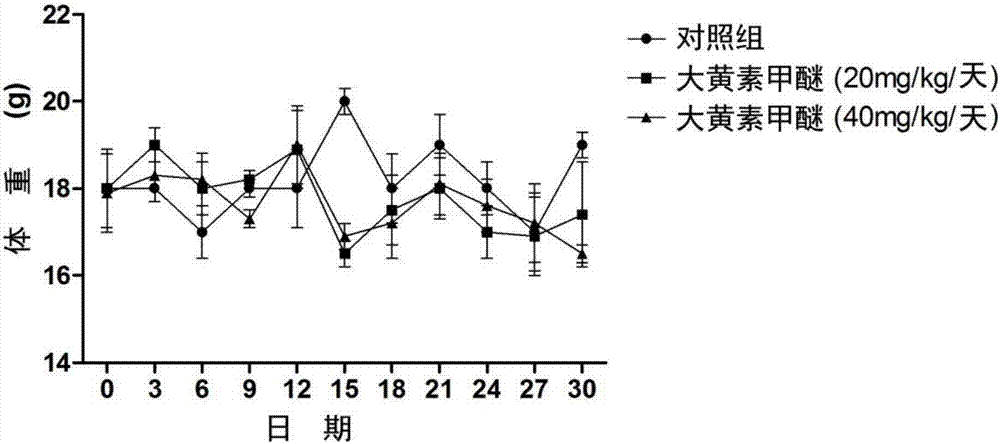
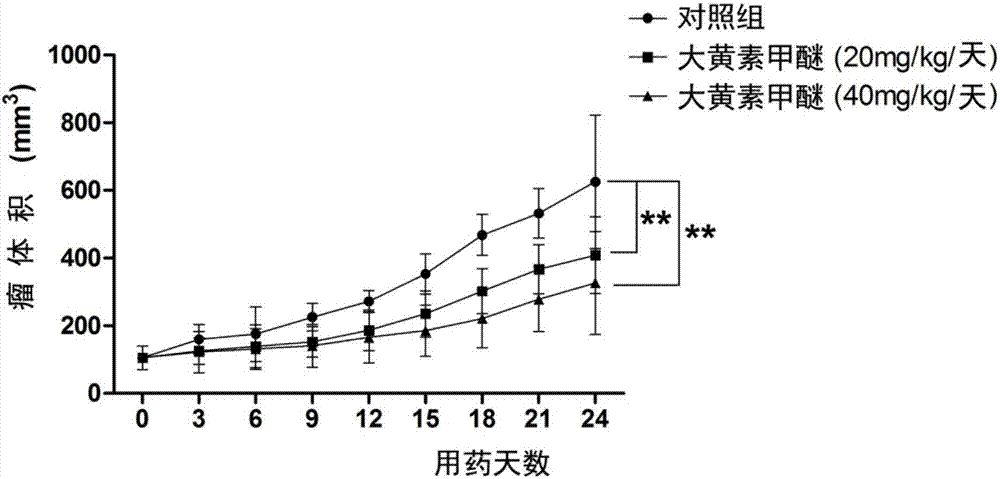
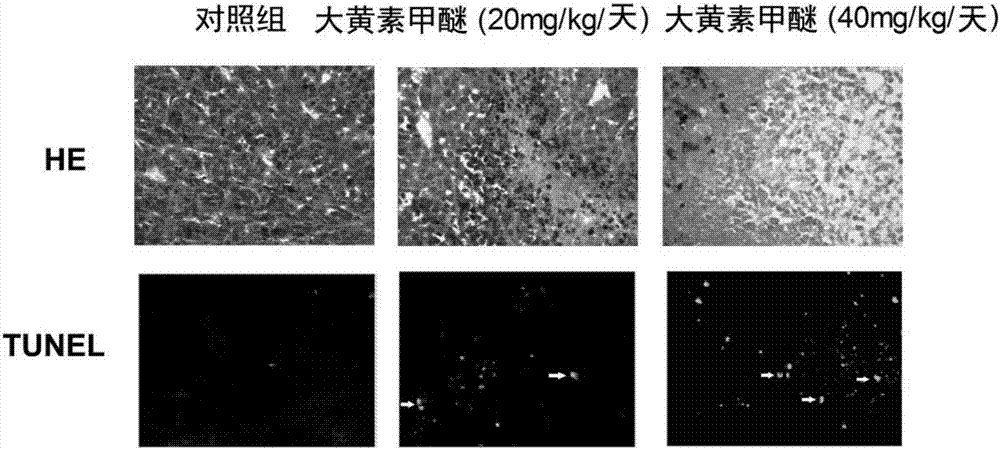
Rheochrysidin and micro-capsule of derivative of rheochrysidin as well as preparation method and application of rheochrysidin
A technology of emodin methyl ether and derivatives, which is applied in the direction of microcapsules, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of low bioavailability and poor drug absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] (1) Take 300 mg of emodin methyl ether powder, disperse and dissolve it in 5 ml of cod-liver oil, and ultrasonically assist it as an oil phase solution;
[0099] (2) adopting 3% sodium alginate aqueous solution as the aqueous phase solution;
[0100] (3) The ratio of water to oil is 8:1;
[0101] (4) Slowly add the oil phase solution into the water phase solution at 23° C., and stir continuously with a magnetic stirrer until an emulsion is formed. The viscosity of the emulsion is relatively high, the time for mixing to form milk is prolonged, and the difficulty of dripping increases;
[0102] (5) Continuously instill the emulsion to 4% concentration of CaCl with a No. 5 needle syringe at a rate of 40 drops / min 2 In the solution, the magnetic stirrer was continuously stirred during the period to form a vortex, equilibrated for 1 hour, filtered, and washed twice with distilled water to form wet microcapsules;
[0103] (6) 1% chitosan solution adjusts pH=6;
[0104] (7)...
Embodiment 2
[0107] (1) Take 300 mg of emodin methyl ether powder, disperse and dissolve it in 5 ml of cod-liver oil, and ultrasonically assist it as an oil phase solution;
[0108] (2) adopting 2% sodium alginate aqueous solution as the aqueous phase solution;
[0109] (3) The ratio of water to oil is 9:1;
[0110] (4) Slowly add the oil phase solution into the water phase solution at 23°C, and stir continuously with a magnetic stirrer until an emulsion is formed;
[0111] (5) Continuously instill the emulsion to 4% concentration of CaCl with a No. 5 needle syringe at a rate of 60 drops / min 2 In the solution, the magnetic stirrer was continuously stirred during the period to form a vortex, equilibrated for 1 hour, filtered, and washed twice with distilled water to form wet microcapsules;
[0112] (6) 1% chitosan solution adjusts pH=6;
[0113] (7) Put the wet microcapsules into the chitosan solution and incubate for 40 minutes, rinse with distilled water twice, and dry naturally to obt...
Embodiment 3
[0116] (1) Take 2000 mg of emodin methyl ether powder, disperse and dissolve it in 35 ml of cod liver oil, and ultrasonically assist it as an oil phase solution;
[0117] (2) adopting 2% sodium alginate aqueous solution as the aqueous phase solution;
[0118] (3) The ratio of water to oil is 10:1;
[0119] (4) Slowly add the oil phase solution into the water phase solution at 25°C, and stir continuously with a magnetic stirrer until an emulsion is formed;
[0120] (5) Continuously drip the emulsion to 3% concentration of CaCl with a No. 5 needle syringe at a rate of 60 drops / min 2In the solution, the magnetic stirrer was continuously stirred during the period to form a vortex, equilibrated for 1 hour, filtered, and washed twice with distilled water to form wet microcapsules;
[0121] (6) 1% chitosan solution adjusts pH=6;
[0122] (7) Put the wet microcapsules into the chitosan solution and incubate for 40 minutes, rinse with distilled water twice, and dry naturally to obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com