A kind of soluble albumin nanoparticle preparation for injection and preparation method thereof
An albumin nanoparticle and injection technology, which is applied in the field of pharmaceutical preparations, can solve the problems of increasing toxic side effects, requiring frequent administration, and low oral bioavailability, avoiding gastrointestinal toxic side effects and avoiding high bioavailability. Low, reduced systemic toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
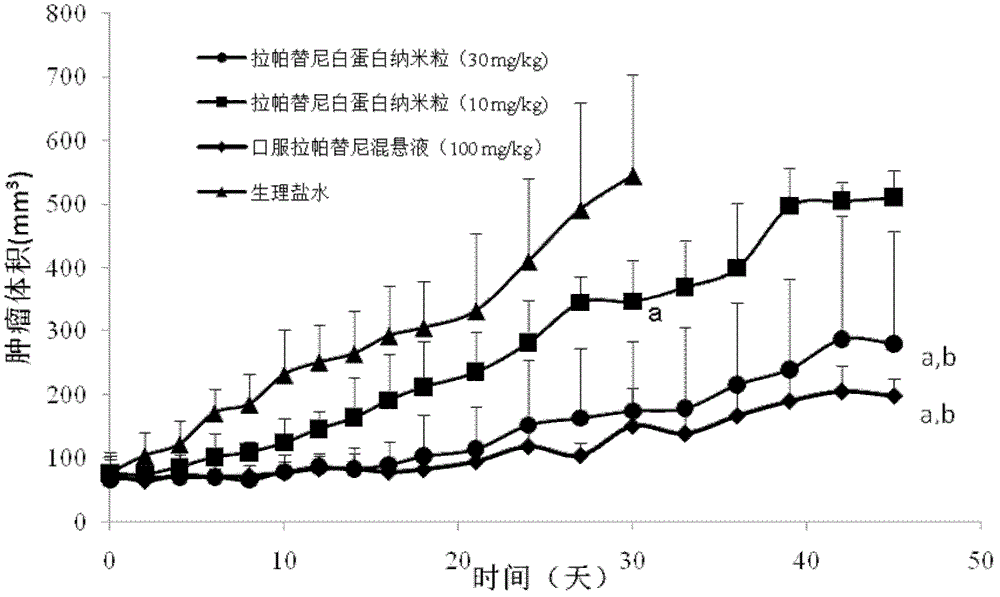
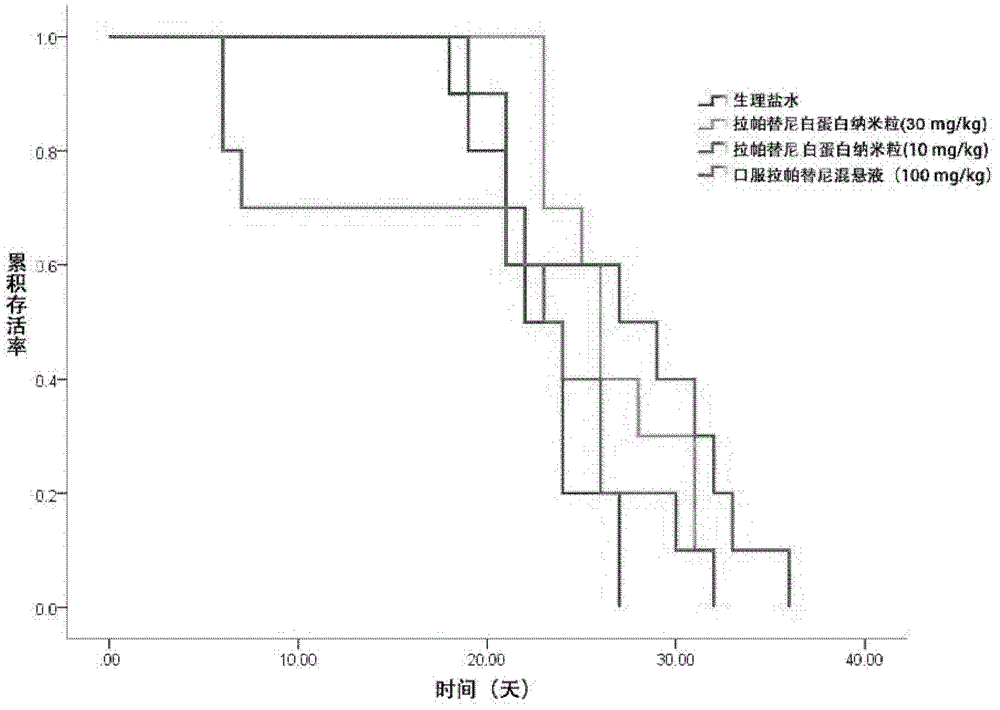
[0040] Embodiment 1, the prescription process of lapatinib albumin nanoparticles
[0041] Table 1 Ratio (mg) of each component in the albumin nanoparticles formulation loaded with lapatinib
[0042]
[0043] Dissolve lapatinib in prescriptions 1 to 30 in Table 1 in an appropriate amount of ethanol-water (6:1 to 2:1) mixed solvent or other suitable solvents, and dissolve phospholipids in an appropriate amount of dichloromethane. After mixing, it is used as the oil phase; dissolve albumin and antioxidants in an appropriate amount of deionized water (water phase), drop the oil phase into the magnetically stirred water phase at room temperature or in an ice-water bath, and continue to stir for a certain period of time, then 40 ° C Ethanol and dichloromethane were removed by rotary evaporation in a water bath to obtain lapatinib-loaded albumin nanoparticles.
Embodiment 2
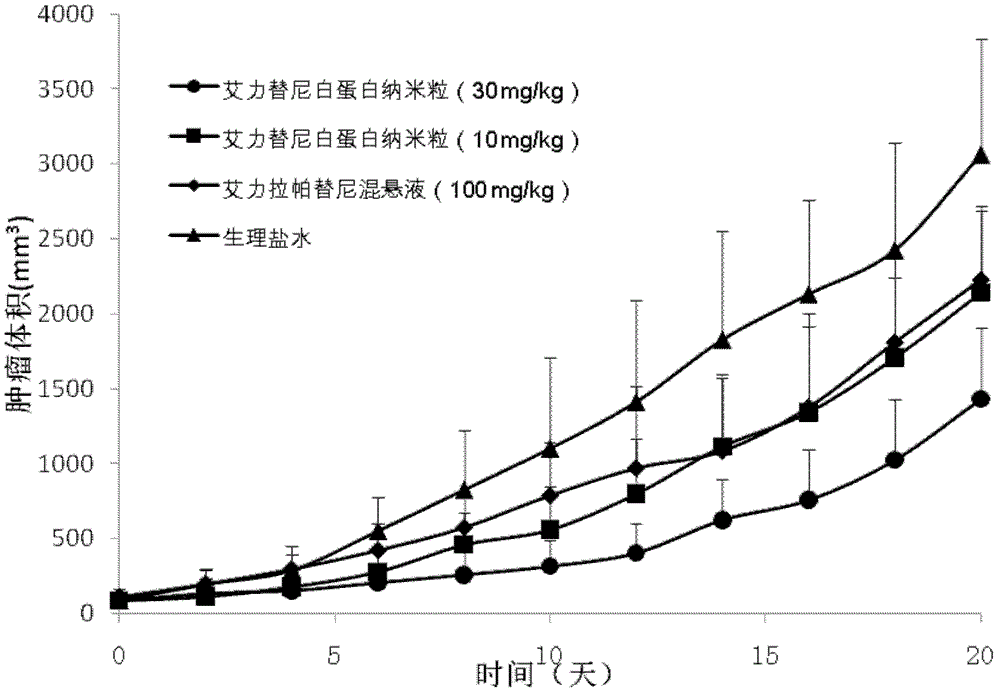
[0044] Embodiment 2, the prescription process of allitinib albumin nanoparticles
[0045] Table 2 The proportion of each component in the albumin nanoparticle formulation loaded with allitinib (mg)
[0046]
[0047] Dissolve allitinib in prescriptions 1-30 in Table 2 in an appropriate amount of ethanol-water (6:1-2:1) mixed solvent or other suitable solvents, dissolve phospholipids in an appropriate amount of dichloromethane, and mix the two Finally, as the oil phase; dissolve albumin and antioxidants in an appropriate amount of deionized water (water phase), drop the oil phase into the magnetically stirred water phase at room temperature or in an ice-water bath, and continue stirring for a certain period of time, then 40 ° C water bath Ethanol and dichloromethane were removed by rotary evaporation to obtain allitinib-loaded albumin nanoparticles.
Embodiment 3
[0048] Embodiment 3, the prescription process of gefitinib albumin nanoparticles
[0049] Table 3 The ratio (mg) of each component in the albumin nanoparticle prescription loaded with gefitinib
[0050]
[0051] Dissolve gefitinib in prescriptions 1-30 in Table 3 in an appropriate amount of ethanol-water (6:1-2:1) mixed solvent or other suitable solvents, dissolve phospholipids in an appropriate amount of dichloromethane, and mix the two Finally, as the oil phase; dissolve the albumin and antioxidants in an appropriate amount of deionized water (water phase), drop the oil phase into the magnetically stirred water phase at room temperature or in an ice-water bath, and continue stirring for a certain period of time. Then the ethanol and dichloromethane were removed by rotary evaporation in a water bath at 40° C. to obtain albumin nanoparticles loaded with gefitinib.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com