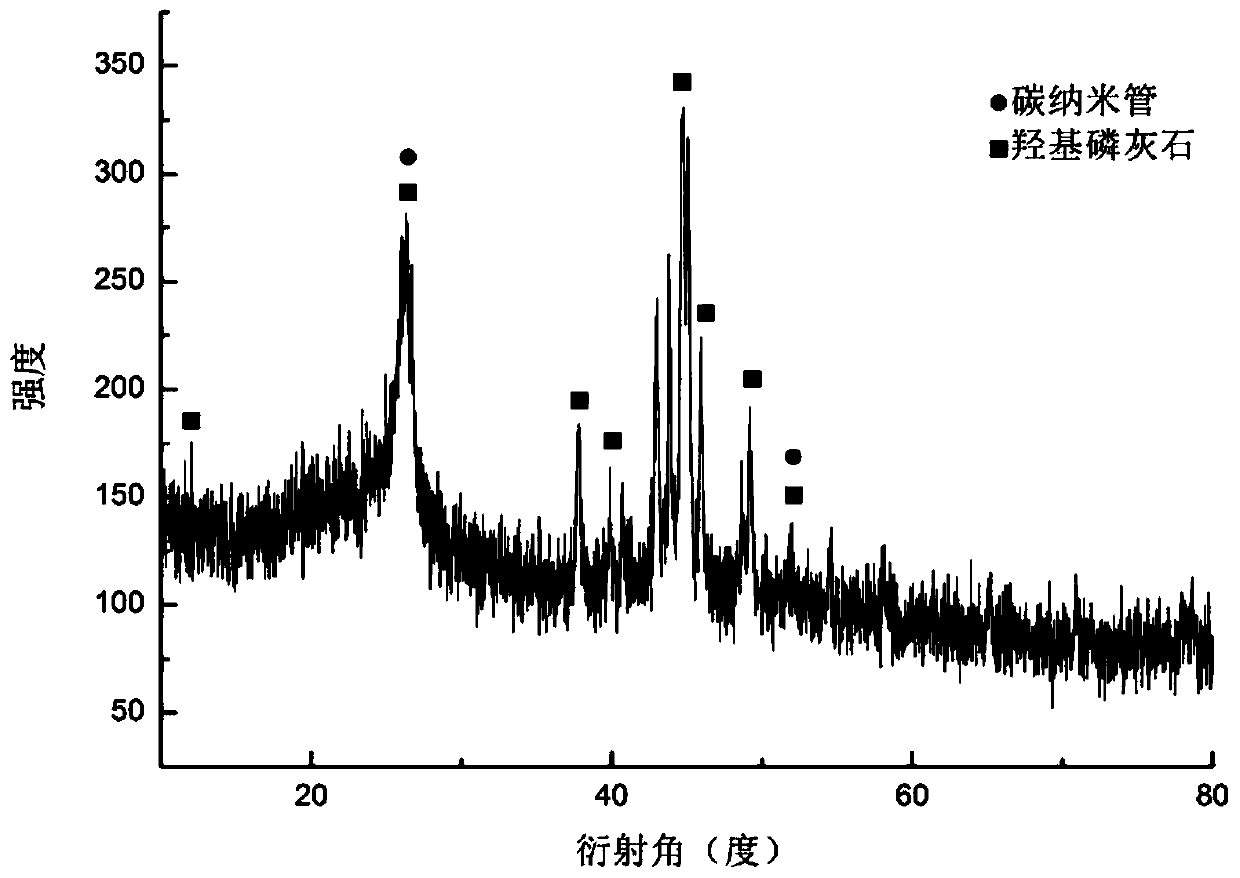
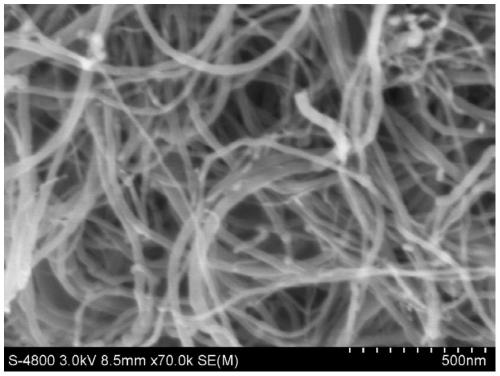
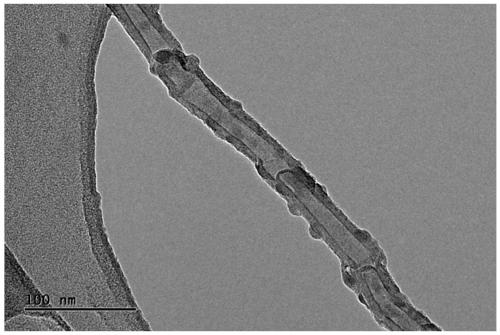
Preparation method of collagen-coated carbon nanotube composite material
A technology of composite materials and carbon nanotubes, applied in the direction of carbon compounds, chemical instruments and methods, medical preparations of non-active ingredients, etc. Poor stability and other problems, to achieve high drug loading, avoid agglomeration, and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The first step is to prepare carbon nanotube-hydroxyapatite composite powder:
[0029] Weigh the required mass of ferric chloride hexahydrate and hydroxyapatite particles with a particle size of 10nm at a mass ratio of 0.55:1, and mix the weighed The hydroxyapatite particles were added to deionized water to form a hydroxyapatite suspension with a molar concentration of 0.01mol / L, and then the weighed ferric chloride hexahydrate was added to the above-mentioned hydroxyapatite suspension, and stirred 2h, ferric chloride is uniformly impregnated in hydroxyapatite to obtain suspension I, suspension I: 25% (mass percentage) ammonia water=100:1 in volume ratio, add 25% (mass percentage) to above suspension I percentage) of ammonia water, and continue to stir for 1 hour to obtain suspension II, place the formed suspension II in an ultrasonic disperser, and disperse ultrasonically at a frequency of 20 kHz for 40 min to fully react ferric chloride and ammonia water to form Fe(OH...
Embodiment 2
[0039] The first step is to prepare carbon nanotube-hydroxyapatite composite powder:
[0040] Weigh the required mass of ferric chloride hexahydrate and hydroxyapatite particles with a particle size of 40nm in a mass ratio of 1.2:1, and mix the weighed ferric chloride hexahydrate with a mechanical stirrer at a speed of 250r / min. The hydroxyapatite particles were added to deionized water to form a hydroxyapatite suspension with a molar concentration of 0.15mol / L, and then the weighed ferric chloride hexahydrate was added to the above-mentioned hydroxyapatite suspension, and stirred 3h, ferric chloride is uniformly impregnated in the hydroxyapatite to obtain the suspension I, the suspension I: 25% (mass percentage) ammonia water=60:1 in the volume ratio, add 25% (mass percentage) to the above suspension I percentage) of ammonia water, and continue to stir for 2.5 hours to obtain suspension II. Place the formed suspension II in an ultrasonic disperser, and ultrasonically disperse...
Embodiment 3
[0046] The first step is to prepare carbon nanotube-hydroxyapatite composite powder:
[0047] Weigh the required mass of ferric chloride hexahydrate and hydroxyapatite particles with a particle size of 60nm according to the ratio of 1.75:1 in mass ratio, and under the condition of stirring at a speed of 400r / min with a mechanical stirrer, the weighed The hydroxyapatite particles were added to deionized water to form a hydroxyapatite suspension with a molar concentration of 0.3mol / L, and then the weighed ferric chloride hexahydrate was added to the above-mentioned hydroxyapatite suspension, and stirred 4h, ferric chloride is uniformly impregnated in hydroxyapatite to obtain suspension I, suspension I: 25% (mass percentage) ammonia water=20:1 in volume ratio, add 25% (mass percentage) to above-mentioned suspension I percentage) of ammonia water, and continue to stir for 4 hours to obtain suspension II, place the formed suspension II in an ultrasonic disperser, and disperse it by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com