Astilbin derivative and preparation method thereof
A technology for fucoside derivatives and compounds, applied in the field of astilbin derivatives and their preparation, can solve the problems of limited effect and the like, and achieve the effects of simple operation, reliable process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
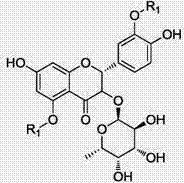
[0041] A preparation method of astilbin derivatives, comprising the following steps:
[0042] A. Dissolving the raw material astilbin AB with 2 to 3 parts by weight in an organic solvent, the organic solvent is N,N-dimethylformamide, tetrahydrofuran or N,N-dimethylacetamide; Under the action, add 0.8~1.2 parts by weight of Bian chlorine or Bian bromine to carry out the substitution reaction, the temperature of the substitution reaction is 50~70°C, the reaction time is 5~7h, and the catalyst is 1~2 parts by weight of K 2 CO3, KI of 0.1-0.5 parts by weight. After the reaction, add water, extract and refine through the extractant to obtain the white solid intermediate AB-N-1; the extractant is ethyl acetate, dichloromethane or chloroform; the refinement is: the organic solvent The extracted organic phase was sequentially acid-washed, washed with water, dewatered, and spin-dried under reduced pressure to obtain white solid intermediate AB-N-1. Among them, dilute hydrochloric aci...
Embodiment 1
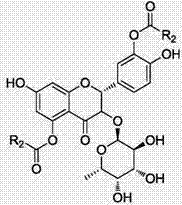
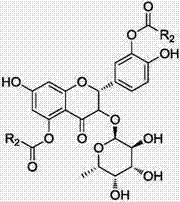
[0047] Embodiment 1, as figure 1 Shown, the preparation method of astilbin derivative, comprises the following steps:
[0048] A, the preparation of AB-N-1
[0049] Add 5.2g of AB raw material to the single-necked bottle, dissolve in 30mL DMF, add anhydrous K 2 CO 3 4g, KI 0.8g, add 6.86mL benzyl chloride, stir and react at 50°C for 7h. After the reaction, add 50mL water to the system, extract with chloroform (30mL×3), wash the organic phase with dilute HCl (20mL×3), wash with water (20mL×3), wash with saturated NaCl (20mL×3), and depressurize After spin-drying, 3.54 g of white solid was obtained, with a yield of 35%.
[0050] B. Preparation of AB-N-2
[0051] Add 0.24g of AB-N-1 to the reaction flask, dissolve it with 5mL of dichloromethane, add 0.15g of Boc-glycine, then add 0.05g of DMAP, 0.1g of DCC, and stir at 20°C for 20h. After the reaction, the reaction solution was suction filtered, the filtrate was spin-dried, 10 mL of water was added, extracted with dichloro...
Embodiment 2
[0056] Embodiment 2, as figure 1 Shown, the preparation method of astilbin derivative, comprises the following steps:
[0057] A, the preparation of AB-N-1
[0058] Add 4g of raw material AB to the single-necked bottle, dissolve in 20mL tetrahydrofuran, add anhydrous K 2 CO 3 3.5 g, KI 0.5g, add 10.97mL benzyl chloride, stir and react at 70°C for 5h. After the reaction, add 50mL water to the system, extract with ethyl acetate (30mL×3), wash the organic phase with dilute HCl (20mL×3), wash with water (20mL×3), wash with saturated NaCl (20mL×3), and depressurize After spin-drying, 4.05 g of white solid was obtained, with a yield of 38%.
[0059] B. Preparation of AB-N-2
[0060] Add AB-N-10.5g to the reaction flask, dissolve in 5mL THF, add 2.62g Boc-leucine, then add 0.05g DMAP, 0.1g DCC, and stir at 35°C for 18h. After the reaction, the reaction solution was suction filtered, the filtrate was spin-dried, 10 mL of water was added, extracted with chloroform (20 mL×3), the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com