Bipyridine derivative with substituted multiple functional groups and method for preparing bipyridine derivative
A technology of multifunctional groups and derivatives, which is applied in the field of preparation of bipyridine derivatives, can solve the problems of high and low yields of 2,2'-bipyridine, achieve simple and easy synthesis steps, simple synthesis methods, The effect of efficient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of 4-amino-3-nitro-2-chloropyridine
[0047] (1) 4-piperidinyl-3-nitro-2-chloropyridine
[0048]
[0049] In a 50 mL three-necked flask, add 4-piperidinyl-3-nitro-2-hydroxypyridine (1.30 g, 5.8 mmol), phosphorus oxychloride (15 mL), under nitrogen protection, heat to 110 °C, keep 5 hours. After the reaction was completed, cool to room temperature, slowly pour into crushed ice (50 mL), then adjust the pH of the solution to 3~4 with sodium hydroxide, extract with dichloromethane (50 mL×3), and dry over anhydrous magnesium sulfate. Column chromatography (silica gel H, ethyl acetate:petroleum ether (v / v) = 1:4) gave a yellow solid (0.98 g, 70%). Melting point: 78-80°C.
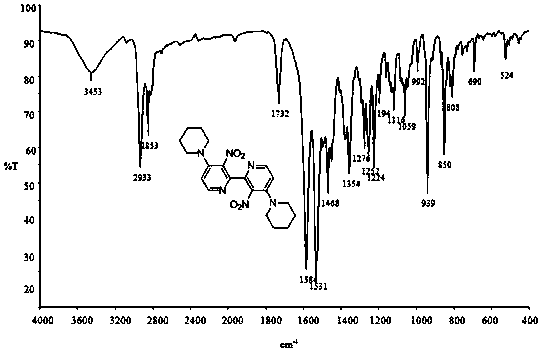
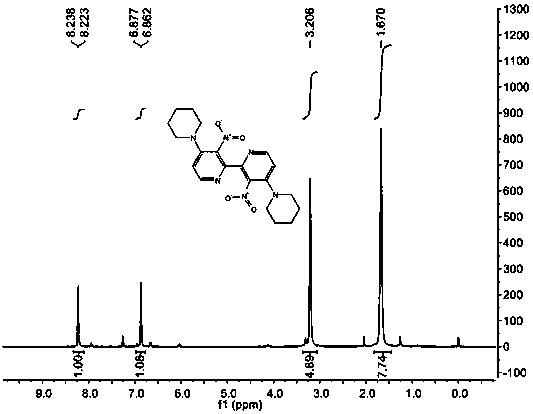
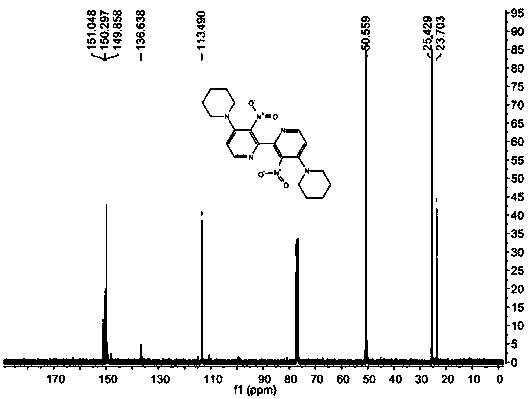
[0050] IR (KBr): ν max 2944, 2926, 2885 (s, C-H), 1523, 1352 (s, N-O) cm -1 ; 1 HNMR (400 MHz, CDCl 3 ): δ 1.67 (m, 6H), 3.21 (m, 4H), 6.80 (d, J = 6.0 Hz, 1H), 8.07 (d, J = 6.0 Hz, 1H); 13 C NMR (100 MHz, CDCl 3 ): δ 23.66, 25.47, 50.37, 112.82, 136.68, 144.13, 149.26, 150.75; ESI...
Embodiment 2
[0060] Synthesis of 4-amino-3-nitro-2-bromopyridine
[0061] (1) 4-piperidinyl-3-nitro-2-bromopyridine
[0062]
[0063] In a 100 mL three-necked flask, add 3-nitro-2,4-dibromopyridine (2.20 g, 7.8 mmol), dichloromethane (20 mL), and then sequentially add triethylamine (1.58 g, 15.6 mmol), piperidine Pyridine (0.70 g, 8.2 mmol), stirred at room temperature for 0.5 hours. After the reaction was complete, it was concentrated and separated by column chromatography (silica gel H, ethyl acetate:petroleum ether (v / v) = 1:3) to obtain a yellow solid (1.80 g, 81%). Melting point: 95-96°C.
[0064] IR (KBr): ν max 2945, 2856 (s, C-H), 1529, 1349 (s, N-O) cm -1 ; 1 H NMR (500 MHz, CDCl 3 ): δ 1.66 (m, 6H), 3.21-3.22 (m, 4H), 6.82 (d, J = 6.0 Hz,1H), 8.06 (d, J = 6.0 Hz, 1H); 13 C NMR (125 MHz, CDCl 3 ): δ 23.65, 25.48,50.46, 113.24, 135.20, 139.19 149.67, 150.58; ESI-MS: m / z 286.1 ([M+H] + ).
[0065] (2) 4-Dimethylamino-3-nitro-2-bromopyridine
[0066]
[0067] In...
Embodiment 3
[0074] Synthesis of 4,4'-diamino-3,3'-dinitro-2,2'-bipyridine
[0075] (1) 4,4'-dipiperidinyl-3,3'-dinitro-2,2'-bipyridine
[0076]
[0077] Method A: In a 50 mL three-necked flask, add 4-piperidinyl-3-nitro-2-chloropyridine (0.50 g, 2.1 mmol), activated copper powder (1.34 g, 21 mmol) and N,N-di Methylformamide (5 mL) was heated to 160°C under nitrogen protection and refluxed for about 5 hours. TLC showed that all the raw materials had reacted. After cooling to room temperature, 2 mmol / L ammonia water (10 mL) was added, resulting in a large amount of precipitation. After filtration, the precipitate was transferred to a Soxhlet extractor and extracted with dichloromethane for 24 h to obtain a yellow extract. The filtrate was extracted with dichloromethane (15 mL×3), and the organic phases were combined and dried over anhydrous magnesium sulfate. Two compounds were separated by column chromatography (silica gel H, ethyl acetate:petroleum ether (v / v) = 1:4-1:1).
[0078] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com