Dysprosium complex constructed with 8-hydroxyquinoline acylhydrazone derivative as ligand, and synthesis method and application of same
A technology of hydroxyquinoline acylhydrazone and a synthetic method, which is applied in the field of magnetic materials, can solve problems such as the lack of a unified explanation of the magnetic action mechanism of single-molecule magnets, and achieve the effects of simple synthesis method, low cost, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
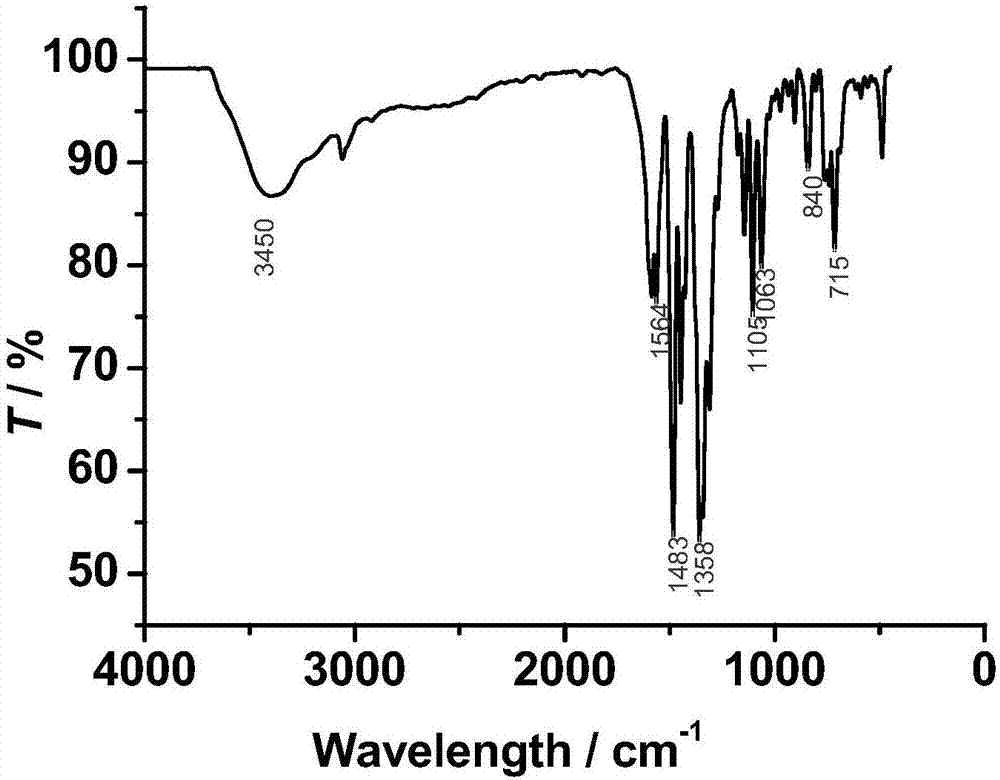
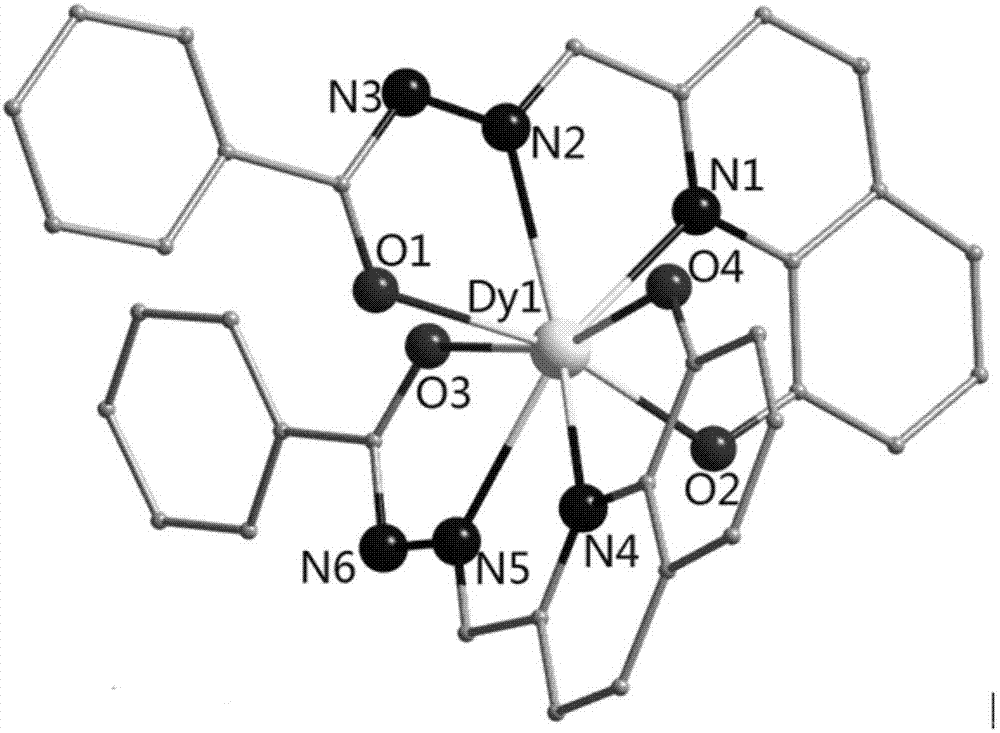
[0039] Take the ligand 2-formyl-8-hydroxyquinoline benzoylhydrazone (0.025mmol, 7.3mg) and Dy(NO 3 ) 3 ·6H 2 O (0.025mmol, 11.4mg), was added to a Pyrex tube with a length of about 18cm that was closed at one end, and 0.6mL of CH 3 OH and 0.6mLH 2 O, add 2 drops of triethylamine dropwise, shake gently (the pH of the solution at this time = 7.8), seal the other end of the tube under the condition of vacuuming, and then keep it at 80°C for 72 hours, take it out, and use Cotton wool wrapped the Pyrex tube tightly and let it cool down to room temperature slowly, and deep red strip crystals were precipitated, with a yield of about 25% (calculated by Dy). Elemental analysis (%) (C 34 h 27 DyN 6 o 6 ), experimental value: C, 52.42, H, 3.62, N, 10.81; theoretical value: C, 52.48, H, 3.50, N, 10.80.
[0040] The product obtained in Example 1 is characterized:
[0041] 1) Infrared characterization:
[0042] Use American Nicolet 360FT-IR type Fourier transform infrared spectrom...
Embodiment 2
[0062] Repeat Example 1, the difference is:
[0063] 1) The ratio of methanol and water in the mixed solvent is 2:1, and the total amount of the mixed solvent remains unchanged;
[0064] 3) Adjust the pH value of the solution to 7.9.
[0065] Take out the Pyrex tube, wrap the Pyrex tube tightly with cotton wool and let it cool down to room temperature slowly, deep red strip crystals are precipitated at the bottom of the Pyrex tube. Yield 27%.
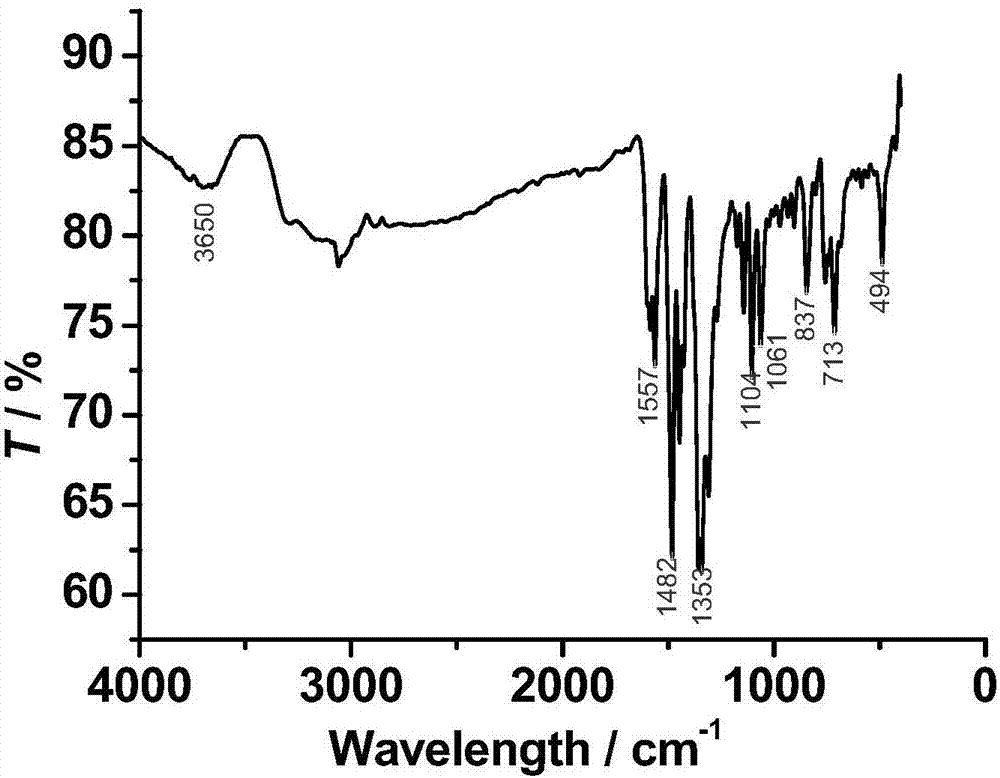
[0066] Structural characterization of the obtained product was carried out, and the product was determined to be the target product [Dy(HL)(L)]·2H 2 O, where L is 2-formyl-8-hydroxyquinoline benzoylhydrazone deprived of a hydroxyl hydrogen atom and a hydrogen atom on the acylhydrazone nitrogen, with two units of negative charge, HL is 2-formyl-8 -Hydroxyquinoline benzoyl hydrazone removes the hydrogen atom of the hydroxyl group, with a unit of negative charge. Characterization of the magnetic properties of the obtained product shows...
Embodiment 3
[0068] Repeat Example 1, the difference is:
[0069] 1) The ratio of methanol and water in the mixed solvent is 1:3, and the total amount of the mixed solvent remains unchanged;
[0070] 2) adjust the pH value of the solution to 8.0;
[0071] 3) Change the reaction temperature to 60°C and the reaction time to 80h.
[0072] Take out the Pyrex tube, wrap the Pyrex tube tightly with cotton wool and let it cool down to room temperature slowly, deep red strip crystals are precipitated at the bottom of the Pyrex tube. Yield 26%.
[0073] Structural characterization of the obtained product was carried out, and the product was determined to be the target product [Dy(HL)(L)]·2H 2O, where L is 2-formyl-8-hydroxyquinoline benzoylhydrazone deprived of a hydroxyl hydrogen atom and a hydrogen atom on the acylhydrazone nitrogen, with two units of negative charge, HL is 2-formyl-8 -Hydroxyquinoline benzoyl hydrazone removes the hydrogen atom of the hydroxyl group, with a unit of negative ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com