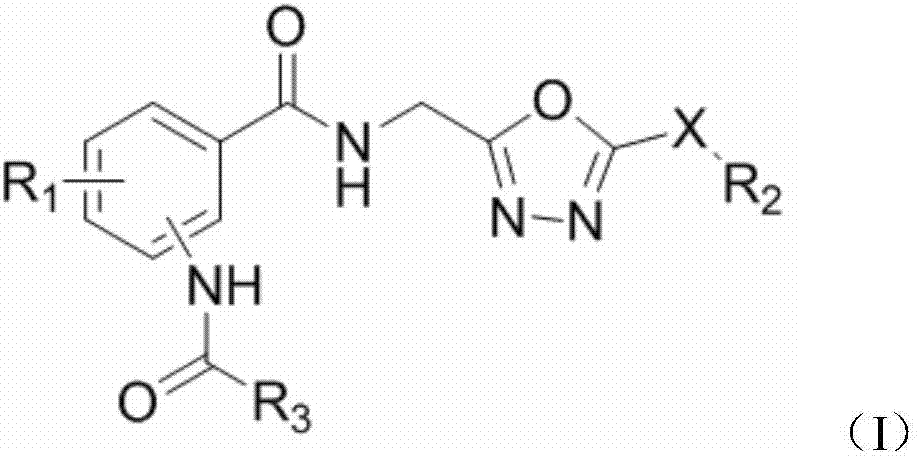
Bisamide compound containing 1, 3, 4-oxiadiazolyl as well as preparation method and application of bisamide compound
An oxadiazole-based compound technology is applied in the field of bisamide compounds and their preparation, and can solve the problems of low control efficiency of antiviral agents, wide damage range, and difficulty in prevention and control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
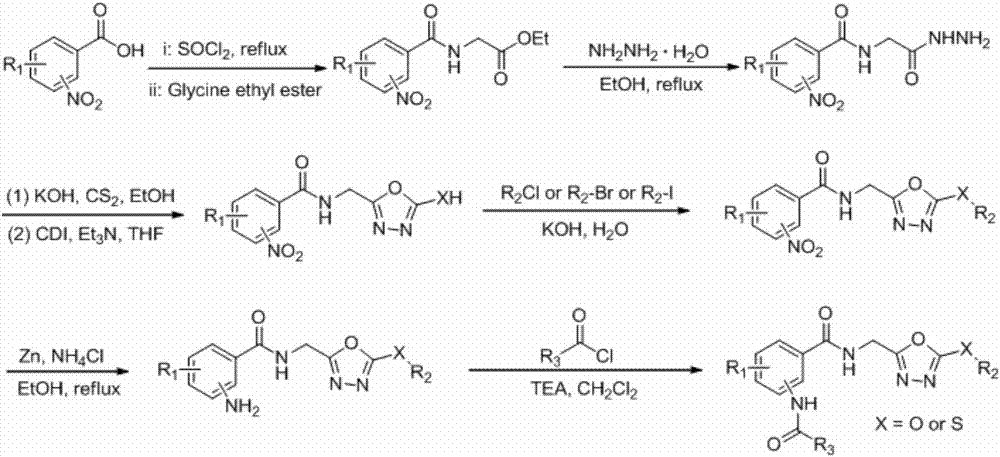
[0021] Example 1 of the present invention: Preparation of the target compound 2-acetylamino-N-(2-methyl-5-(methylsulfide)-1,3,4-oxadiazole)benzamide
[0022]Acetyl chloride (0.09g, 1.14mmol) was added to a solution of 2-amino-N-(2-methyl-5-(methylsulfide)-1,3,4-oxadiazole)benzamide (0.20 g, 0.76mmol) and triethylamine (0.2mL) in 8mL dry tetrahydrofuran solution, react at room temperature for 12 hours, stop the reaction, remove the solvent, add 50mL ethyl acetate, wash 20mL×3 times with water, dry over anhydrous sodium sulfate, remove solution, column chromatography (eluent CH 2 Cl 2 :CH 3 OH=20:1) to obtain a white solid with a yield of 11.4% and a melting point of 143-144°C.
[0023] The synthesis of other target compounds refers to Example 1.
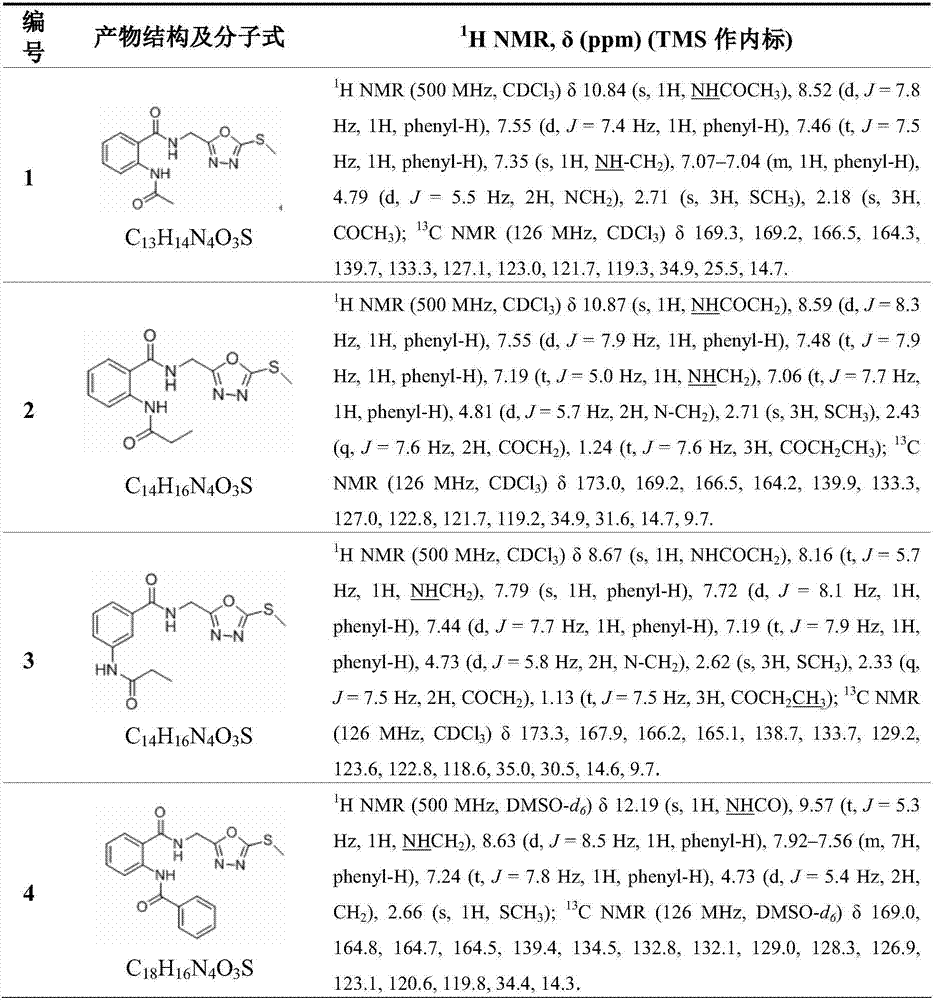
[0024] The structures, H-NMR and C-NMR data of the synthesized bisamide compounds containing 1,3,4-oxadiazolyl are shown in Table 1, and their physical and chemical properties are shown in Table 2.
[0025] H NMR spectrum and car...
Embodiment 2
[0039] Pharmacological Example 2: Antiviral activity test of some target compounds (anti-tobacco mosaic virus)
[0040] The anti-plant virus activity of the compounds was determined by half-leaf spot method. Accurately weigh 3 mg of the test compound in a weighing bottle, and add 60 μL of solvent DMSO to fully dissolve it. Use double distilled water containing 1% Tween 20 to prepare a 500 mg / L compound solution. Take another 250 μL of 2% Ningnanmycin aqueous solution, add 60 μL of solvent DMSO, and 10 mL of double distilled water containing 1% Tween 20 to prepare a 500 mg / L Ningnanmycin solution.
[0041] In vivo therapeutic activity of agents against TMV infection. Select tobacco heart leaves with the same growth, and first dip the virus liquid (concentration is 6×10 -3 mg / mL), artificially rubbed and inoculated on the leaf surface (full leaf) along the direction of its branch veins on the leaves sprinkled with emery. The inoculation intensity of the left and right leaves ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com