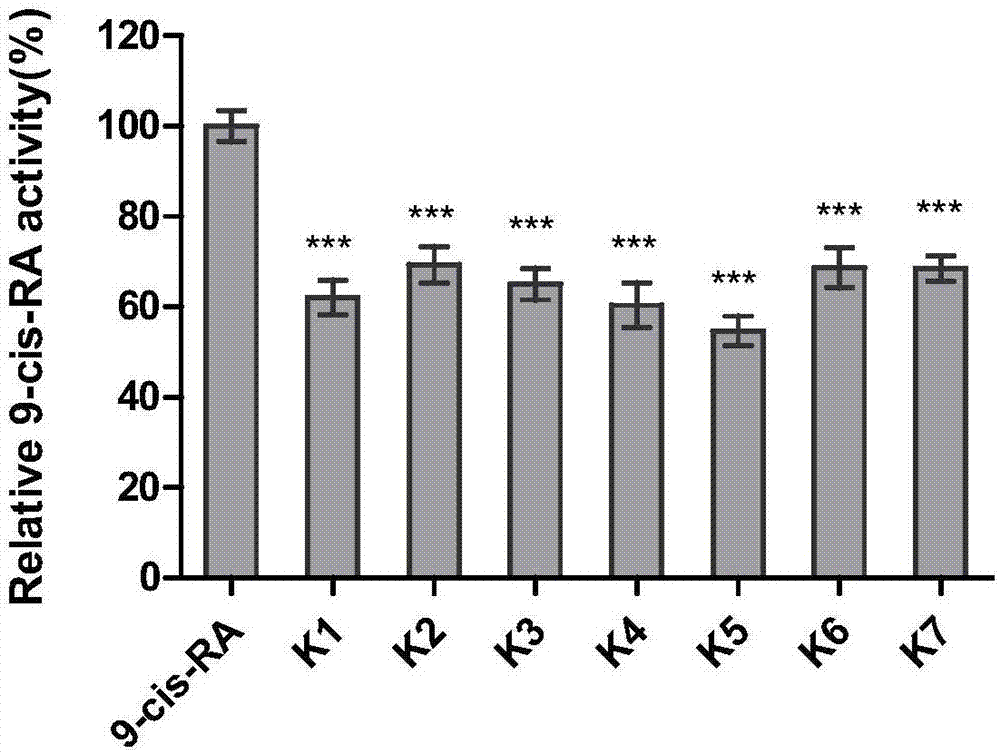
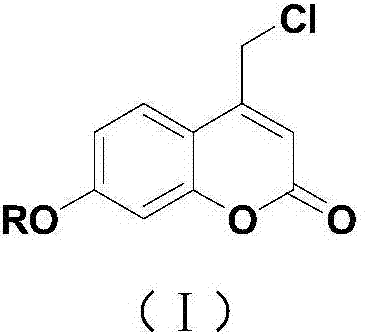
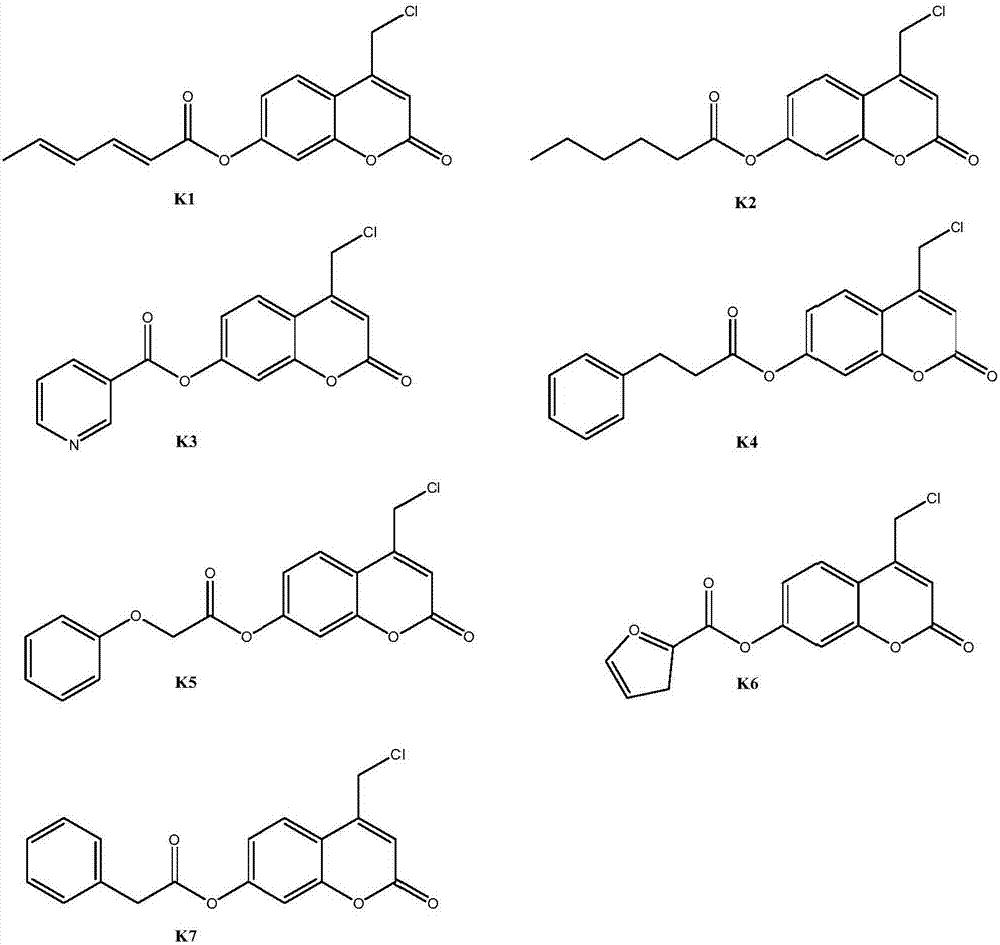
Coumarin esterified derivative with effect of inhibiting transcriptional activity of RXRalpha (Retinoid X Receptor) as well as preparation method and application of coumarin esterified derivative
A technology of derivatives and coumarin, applied in the field of coumarin esterification derivatives and preparation thereof, achieves the effects of short reaction time, less environmental pollution, and inhibition of RXRα transcriptional activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of 4-chloromethyl-7-hydroxycoumarin
[0032] Mix resorcinol (5.5g, 0.05mol), ethyl 4-chloroacetoacetate (9.9g, 0.06mol), anhydrous bismuth chloride (0.8g, 2.5mmol) in a 100mL round bottom flask, and Under solvent conditions, heat and stir at 65°C for 5 hours. After the reaction, add 16 mL of 50% ethanol aqueous solution, continue heating and stirring at 65°C for 10 minutes, cool to room temperature, filter with suction, wash the solid part with water until neutral, and dry to obtain a white solid 7.45 g, namely 4-chloromethyl-7-hydroxycoumarin, the yield is 70%.
[0033] NMR data of 4-chloromethyl-7-hydroxycoumarin:
[0034] 1 H NMR (400MHz, DMSO-d 6 ):δ4.95(s,2H,CH 2 Cl),6.42(s,1H,3-H),6.76(d,1H,J=2.4Hz,8-H),6.83,6.85(dd,1H,J=2.4Hz,J=8.7Hz,6- H),7.67(d,1H,J=8.7Hz,5-H),10.66(s,1H,OH)ppm
Embodiment 2
[0035] Embodiment 2: Preparation of 4-chloromethyl-7-sorboyloxycoumarin (compound K1)
[0036] At 0°C, put sorbic acid (0.224g, 2.0mmol) in a 25mL two-neck round bottom flask, drop 2 drops of DMF, add 10mL of anhydrous dichloromethane (CH 2 Cl 2 ) was dissolved, and oxalyl chloride (0.2mL, 4.0mmol) was added with a disposable syringe; one mouth of the two-necked round-bottomed flask was tightly closed with a rubber stopper, and the other mouth was connected to an exhaust gas absorption device; after 30 minutes in an ice bath, it gradually returned to room temperature, and at room temperature Stir the reaction for 2h, after the reaction is over, distill to dryness under reduced pressure to remove unreacted oxalyl chloride; the product is washed with 2mL CH 2 Cl 2 After dissolving, it was added to 10 mL of CH containing 4-chloromethyl-7-hydroxycoumarin (0.315 g, 1.5 mmol) and triethylamine (0.208 g, 1.8 mmol) with a syringe 2 Cl 2 In the mixed solution, react at room tempera...
Embodiment 3
[0039] Embodiment 3: Preparation of 4-chloromethyl-7-hexanoyloxycoumarin (compound K2)
[0040] At 0°C, put hexanoic acid (0.232g, 2.0mmol) in a 25mL two-neck round bottom flask, drop 2 drops of DMF, add 10mL of CH 2 Cl 2 Dissolve, add oxalyl chloride (0.2mL, 4.0mmol) with a disposable syringe; one mouth of the two-neck round bottom flask is tightly closed with a rubber stopper, and the other mouth is connected to an exhaust gas absorption device; after 30 minutes in ice bath, gradually return to room temperature, and stir at room temperature Reacted for 2h, after the reaction was over, distilled to dryness under reduced pressure to remove unreacted oxalyl chloride; the product was washed with 2mL CH 2 Cl 2 After dissolving, it was added to 10 mL of CH containing 4-chloromethyl-7-hydroxycoumarin (0.315 g, 1.5 mmol) and triethylamine (0.208 g, 1.8 mmol) with a syringe 2 Cl 2 In the mixed solution, react at room temperature for 3 h; after the reaction, adjust the pH value of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com