Method for purifying streptococcus pneumoniae capsular polysaccharide
A technology of Streptococcus pneumoniae and capsular polysaccharide, applied in the field of medicine, can solve the problems of restricting large-scale use, expensive nuclease, etc., and achieves the effects of mild reagents, no damage to the environment and human body, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
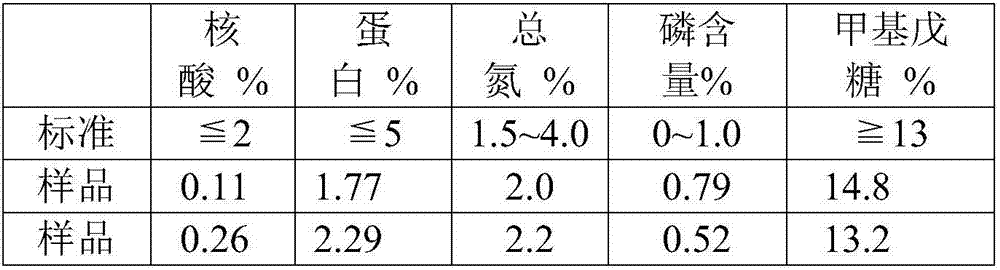
[0043] 1) Take Streptococcus pneumoniae culture solution with serotype 7F, add sodium deoxycholate (DOC) with a final concentration of 0.12%, stir overnight, centrifuge to remove large particles of impurities, and collect the supernatant;
[0044] 2) Use an ultrafiltration membrane with a molecular weight cut-off of 100KDa for ultrafiltration and liquid replacement. Before ultrafiltration, use a 0.45-0.8 μm filter for pre-filtration, use 0.1M phosphate buffer as the ultrafiltration buffer, and the pH value is 7.0 ;
[0045] 3) Adjust the pH of the filtrate obtained after ultrafiltration to 4.0, place it in a cold storage and stir for 30 minutes, and retain the supernatant by high-speed refrigerated centrifugation;
[0046] 4) Adding mass percent concentration to the supernatant is 20% CaCl 2 Mix the aqueous solution to a final concentration of 3% in the system, stir for 10-30 minutes, adjust the pH to about 9.0, place in a cold storage and stir for 30 minutes, and centrifuge at...
Embodiment 2
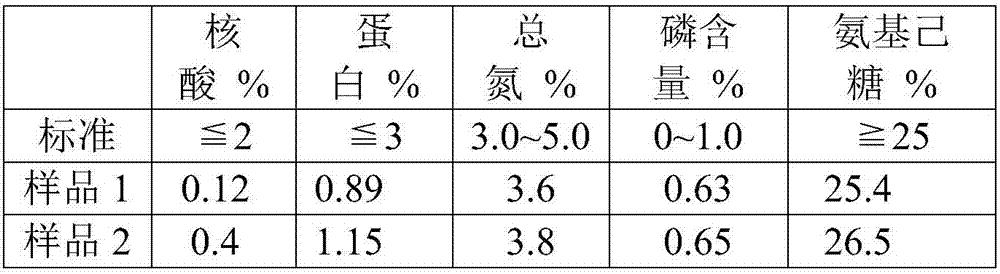
[0049] Take the Streptococcus pneumoniae culture fluid with serotype 12F, add sodium deoxycholate (DOC) with a final concentration of 0.12%, stir overnight, centrifuge to remove large particles of impurities, and collect the supernatant; and use ultrafiltration with a molecular weight cut-off of 100KDa The membrane is used for ultrafiltration and liquid replacement. Before ultrafiltration, use a 0.45-0.8μm filter for pre-filtration. The ultrafiltration buffer uses 0.1M phosphate buffer, and the pH value is about 7.0; adjust the pH of the sample after ultrafiltration to 4.0, placed in the cold storage and stirred for 30 minutes, high-speed refrigerated centrifugation to retain the supernatant; add CaCl with a final concentration of 3% to the supernatant 2 solution, stirred for 10 to 30 minutes, adjusted to a pH of about 9.0, placed in a cold storage and stirred for 30 minutes, high-speed refrigerated and centrifuged to retain the supernatant; then added ethanol to the supernatan...
Embodiment 3
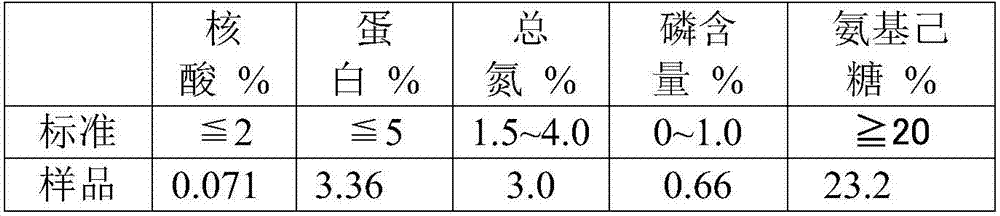
[0051] Take the Streptococcus pneumoniae culture fluid with serotype 14, add sodium deoxycholate (DOC) with a final concentration of 0.12%, stir overnight, centrifuge to remove large particles of impurities, and collect the supernatant; and use ultrafiltration with a molecular weight cut-off of 100KDa The membrane is used for ultrafiltration and liquid replacement. Before ultrafiltration, use a 0.45-0.8μm filter for pre-filtration. The ultrafiltration buffer uses 0.1M phosphate buffer, and the pH value is about 7.0; adjust the pH of the sample after ultrafiltration to 4.0, placed at room temperature and stirred for 30 minutes, high-speed refrigerated centrifugation to retain the supernatant; add a final concentration of 3% CaCl to the supernatant 2 Solution, stirred for 10-30 minutes, adjusted to pH 9.0, placed at room temperature and stirred for 30 minutes, high-speed refrigerated and centrifuged to retain the supernatant; The buffer solution is 0.5M sodium chloride, and then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com