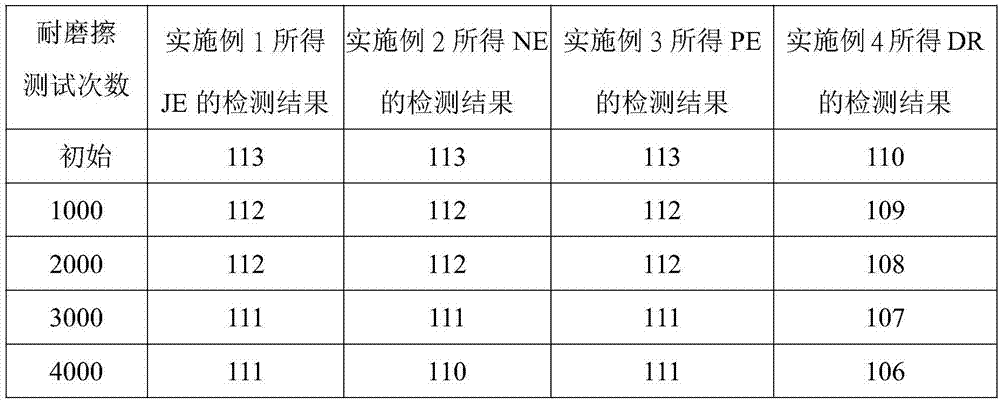
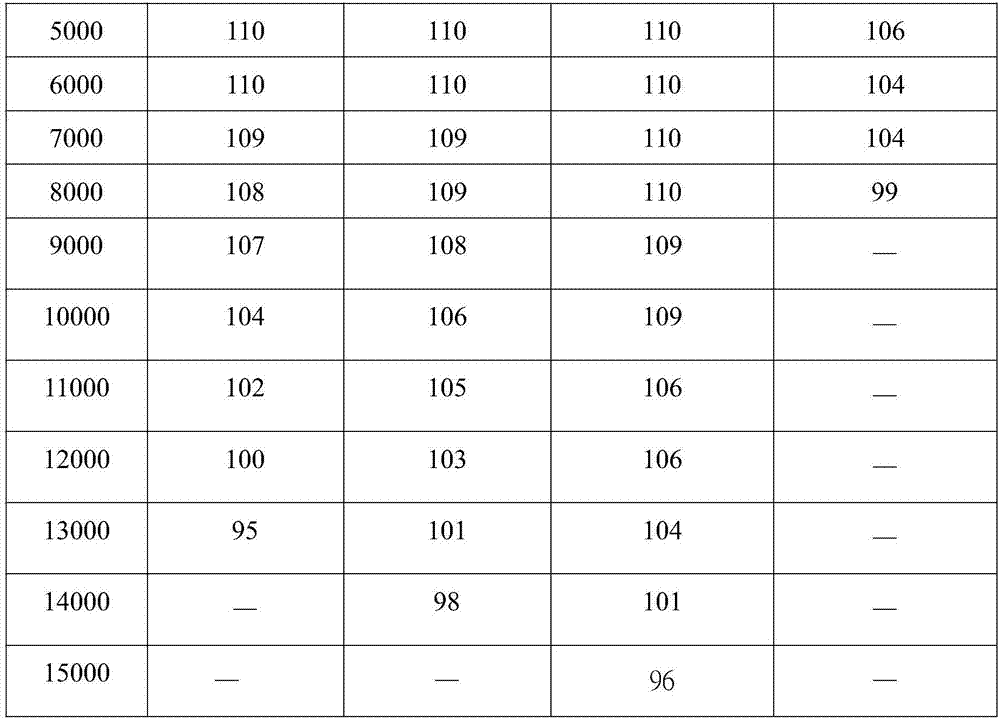
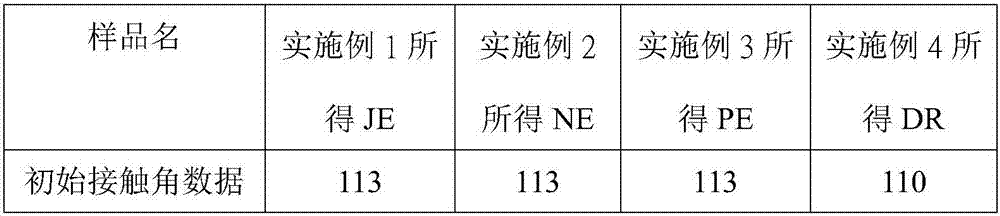
Perfluoropolyether-modified silane compound, surface treatment composition comprising same and thin film
A silane compound, perfluoropolyether technology, applied in polyether coatings, biocide-containing paints, coatings, etc., can solve the problem that the improvement of stain resistance cannot be maximized and the possibility of multiple bonding is reduced , The tightness of the treatment agent segment and the substrate cannot be fully exerted, etc., to achieve the effect of excellent anti-fouling and good smoothness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Synthesis of perfluoropolyether modified silane compound JE
[0027] Step (A-1): Reduction of acyl fluoride ethers to fluoroether alcohols (1-1)
[0028] In a 2.0L four-necked flask equipped with a stirrer, dropping funnel, reflux condenser and thermometer, 250 g of diethylene glycol dimethyl ether and 11.4 g (0.3 mol) of NaBH 4 , and then 525g (0.175 moles) by chemical formula F-(CF 2 CF 2 O) n (CF 2 O) n -CF 2 A fluoropolyperfluorooxirane fluoride ether compound represented by -COF (average molecular weight: 3000) was added dropwise to the above-mentioned raw material mixture at a rate of 10 mL / minute under nitrogen blanketing. After completion of the dropwise addition, the temperature of the liquid phase was raised to about 90° C., and the reaction mixture was allowed to proceed at this reaction temperature for 6 hours. After the reaction, the mixture in the flask was stirred and cooled to below 40°C, then slowly added 525 grams of 10% hydrochloric ...
Embodiment 2
[0049] Example 2 Synthesis of perfluoropolyether modified silane compound NE
[0050] Step (B-2): Reaction of acyl fluoride ether with lithium iodide to generate corresponding fluoroether iodide (2-1)
[0051] Add 164 grams by chemical formula F-(CF 2 CF 2 O) n (CF 2 O) m -CF 2 The fluorine polyperfluoroethylene oxide group represented by -COF (average molecular weight is 3000) and 21.7 grams of lithium iodide, after nitrogen replacement, then reacted at 180 ° C for 10 hours, after cooling to room temperature, After removing the solid matter in the reaction mixture, 160 g of reaction crude product can be obtained. This crude product is found to be the target compound 2-1 of following two structures through NMR analysis: and the mixture of by-product 2-2 (approximate ratio is 90:10):
[0052] CF3 CF 2 O-(CF 2 CF 2 O) m -(CF 2 O) n -CF 2 -I (2-1)
[0053] CF 3 CF 2 O-(CF 2 CF 2 O) m -(CF 2 O) n -CF 2 -H (2-2)
[0054] Using infrared and NMR spectroscopic ...
Embodiment 3
[0082] Example 3 Synthesis of perfluoropolyether modified silane compound PE
[0083] Step (C-3a): Compound (2-5) undergoes silylation reaction with methyldichlorosilane
[0084] In order to prevent the mixture (2-5) having a vinyl group at the end generated by the step (C-2b) of the above synthesis example from deactivating due to the poisoning effect (poison) of the platinum catalyst used in the hydrogen silicon reaction due to the existence of a small amount of iodide, The vinyl mixture (2-5) obtained in the above steps is stirred with powdered zinc powder and 5% acetic acid aqueous solution at room temperature for half an hour before being used in the reaction, and the filtered filtrate is left to stand to separate the lower organic layer (see US patent 5,166,453 ), and washed once with water, and the processed product obtained in this way is decompressed to remove volatile matter for later use.
[0085] The 100 ml four-necked flask equipped with a reflux condenser, a the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com