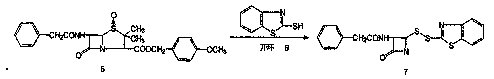
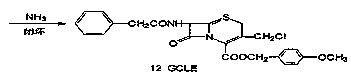
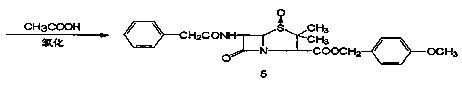
Preparation of 7-phenylacetamide-3-chloromethyl cephalosporanic acid p-methoxybenzyl ester
A technology of chloromethyl cephalosporanic acid and p-methoxybenzyl ester, which is applied in the field of drug synthesis, can solve the problems of high industrial production cost, low GCLE yield, and difficult recovery of mixed solvents, and achieve stable product quality and easy equipment operation The effect of simplicity and ease of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1), the synthesis of p-methoxybenzyl chloride
[0049] 70g of p-methoxybenzyl alcohol (0.508mmol), at 10~15°C, slowly add 100g of 36% hydrochloric acid (0.986mmol) dropwise, keep the reaction at 20°C for 1h, separate the hydrochloric acid layer, and obtain 80g of p-methoxybenzyl chloride .
[0050] (2) Synthesis of penicillin sulfoxide p-methoxybenzyl ester
[0051] Weigh 175g (0.470mol) of penicillin G potassium salt, 8g (0.025mol) of alkylamine bromide, add 500ml of xylene, 60ml of DMF, start stirring, add 80g (0.510mol) of benzyl chloride prepared in the previous step to the bottle, and heat up Insulate and react at 55~60°C for 6~8 hours, lower to room temperature, add 400ml of purified water, separate the water layer, cool down with cold salt water, when the internal temperature is 0~5°C, add 20% peracetic acid dropwise into the flask 220g (0.579mol), dripping in 30 minutes, after oxidation, control the temperature at 0-5°C, stir for 2 hours, filter with suction,...
Embodiment 2
[0057] Synthesis of GCLE
[0058] 3000ml of dioxane, 180g (2.14mol) of sodium bicarbonate, 200g of azetidinone sulfinic acid intermediate (0.336mmol), stirred at 10~15°C, slowly introduced 30g (0.422mol) of chlorine gas, about 3h, According to HPLC analysis, the raw material area accounts for less than 0.5%, stop the chlorine gas flow, filter out sodium bicarbonate, recover dioxane under reduced pressure, add 2000ml methanol to dissolve, maintain the temperature at 0~5℃, add 18g (0.34mol) of sodium methoxide and 900ml Sodium methoxide solution prepared with methanol, react at 0~5°C for 30 minutes after adding, add 10% hydrochloric acid to adjust the pH of the system to 4~6, cool down to 0~5°C, stir for 1 hour, filter, wash with water and methanol, and dry. Get GCLE120g. Yield 73%, content ≥ 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com