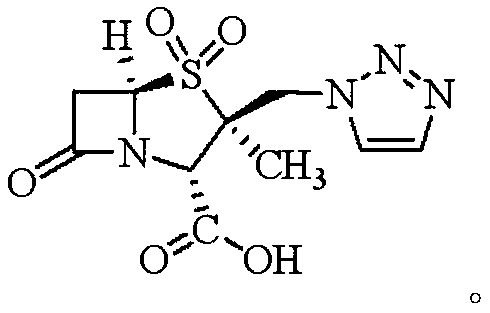
A kind of synthetic method of tazobactamic acid
A technology of tazobactam acid and a synthesis method, applied in directions such as organic chemistry, can solve the problems of long synthesis process steps, many by-products, complicated processes, etc., and achieves the advantages of improving yield and quality, improving quality and reducing reaction temperature. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
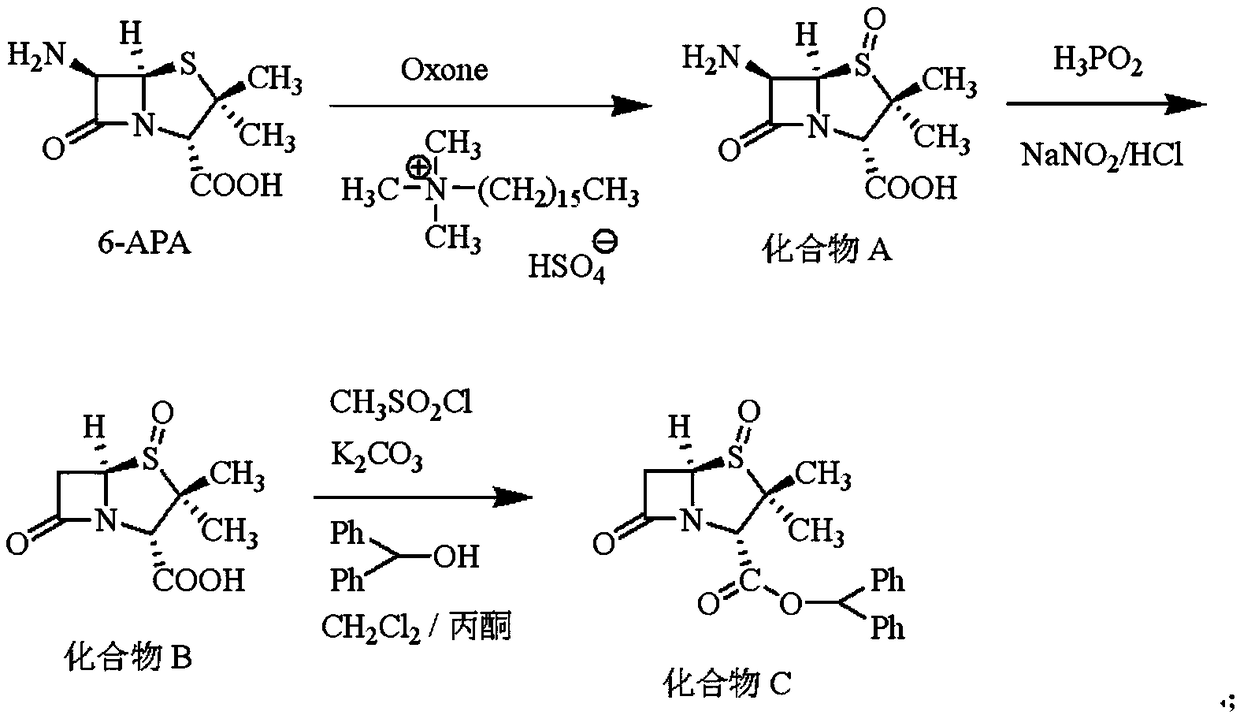
Embodiment 1
[0031] The synthesis of embodiment 1 compound C
[0032] Put 300mL water, 100mL acetone, 21.63g (0.10mol) 6-APA, 3.82g (0.01mol) hexadecyl trimethylammonium hydrogen sulfate into a 1000mL four-necked flask, stir and cool down to 0~5 ℃, time-consuming Add 33.81g (0.055mol) Oxone (2KHSO) in batches for 30min 5 ·KHSO 4 ·K 2 SO 4 Compound salt), react at 0-5° C. for 2 h, and when HPLC detects that the 6-APA residue is less than 1%, the reaction is terminated to obtain compound A.
[0033] Cool the above reaction system to -20~-10℃, add 50g hydrochloric acid with a mass fraction of 36%, add dropwise 26.4g hypophosphorous acid aqueous solution with a mass fraction of 50% (hypophosphorous acid is 0.20mol), and then dropwise add 46.0 mol of hypophosphorous acid. g 30% aqueous sodium nitrite solution (0.20 mol of sodium nitrite) was dripped, and incubated for 2 hours. When the residual compound A was detected by HPLC to be less than 1%, the reaction was terminated to obtain compoun...
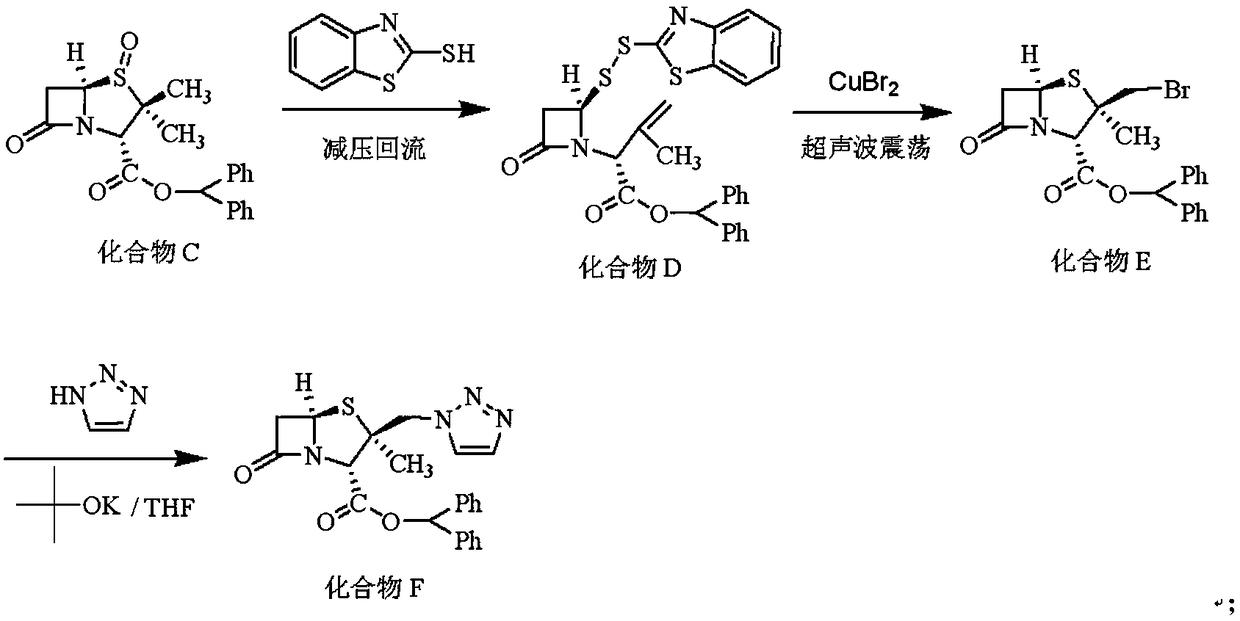
Embodiment 2
[0037] The synthesis of embodiment 2 compound F
[0038] Install a water separator on the 500mL four-neck bottle with a serpentine condenser tube, install a vacuum tube connected to the vacuum pump and a valve for adjusting the air volume on the upper part of the serpentine condenser tube, and put 200mL into the four-neck bottle. Toluene, 38.35g (0.10mol) of compound C, 16.73g (0.10mol) of 2-mercaptobenzothiazole, by adjusting the suction volume, controlling the internal temperature to be 70-80°C, and refluxing under reduced pressure for 3h, to be detected by HPLC that the residue of compound C is less than At 1%, the reaction was terminated. Concentrate to dryness under reduced pressure, add 200 mL of tetrahydrofuran and stir to dissolve, filter off insoluble matter, and concentrate the filtrate to dryness under reduced pressure to obtain Compound D as a white foam.
[0039] Dissolve compound D with 200 mL of dichloromethane, insert an ultrasonic vibrating rod into the react...
Embodiment 3
[0050] The synthesis of embodiment 3 tazobactam acid
[0051] Put 100mL dichloromethane, 50mL acetone, 43.45g (0.10mol) compound F, 16.33g mass fraction of 50% hydrogen peroxide into a 500mL four-neck flask (hydrogen peroxide is 0.24mol), 0.024g sodium polyphosphate (50 % 0.15% of the weight of hydrogen peroxide), cooled to -5~0°C, added 24.50g (0.24mol) acetic anhydride dropwise, exothermic, temperature controlled at 0~5°C, dripping completed, kept for 4h, to be detected by HPLC that the residue of compound F was less than At 1%, the reaction was terminated. 200 mL of 5% sodium sulfite aqueous solution was added to the reaction system, stirred for 10 min, left to stand for layers, the aqueous layer was extracted with 100 mL of dichloromethane, and about 300 mL of the dichloromethane layers were combined to obtain a solution of compound G in dichloromethane.
[0052] Transfer the methylene chloride solution of compound G of the previous step into a 1000mL four-necked flask, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com