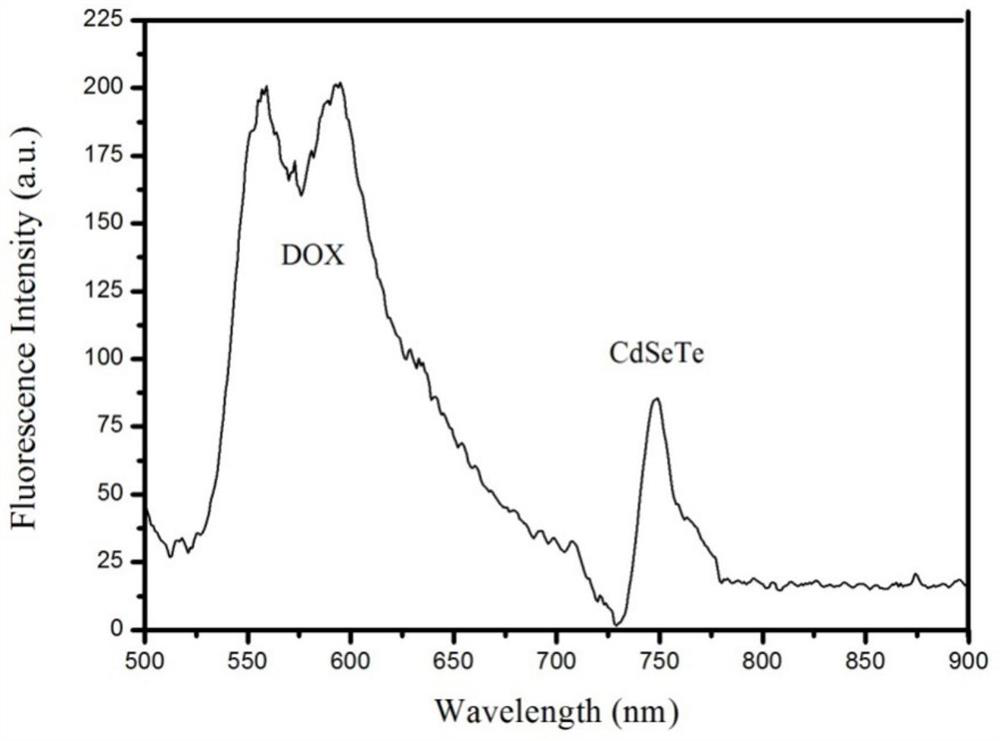
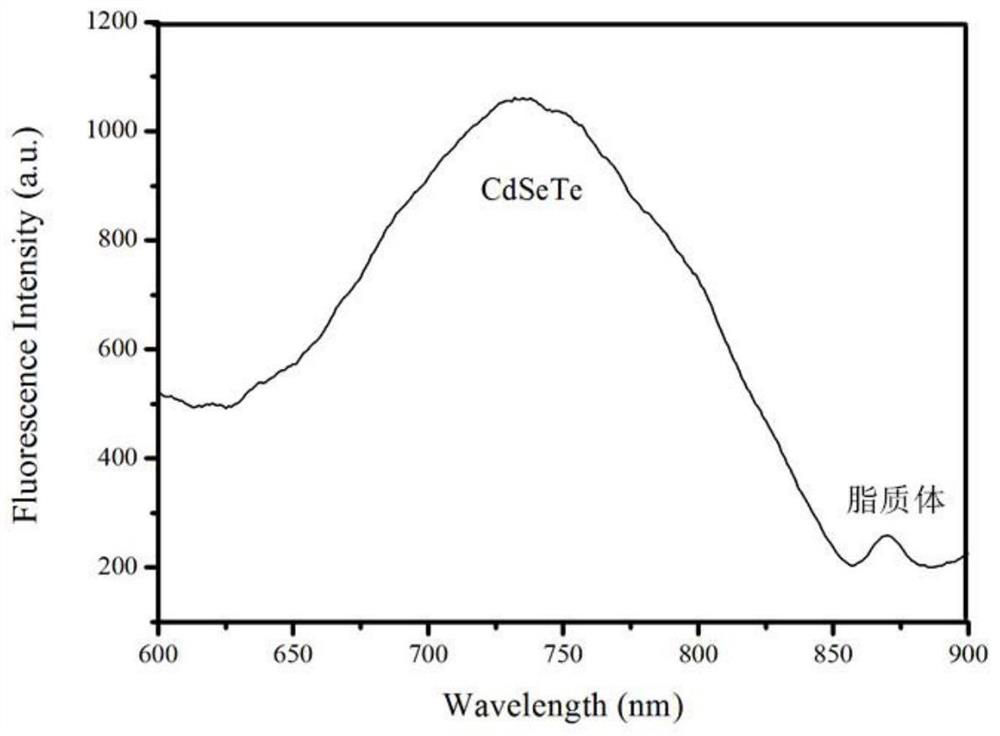
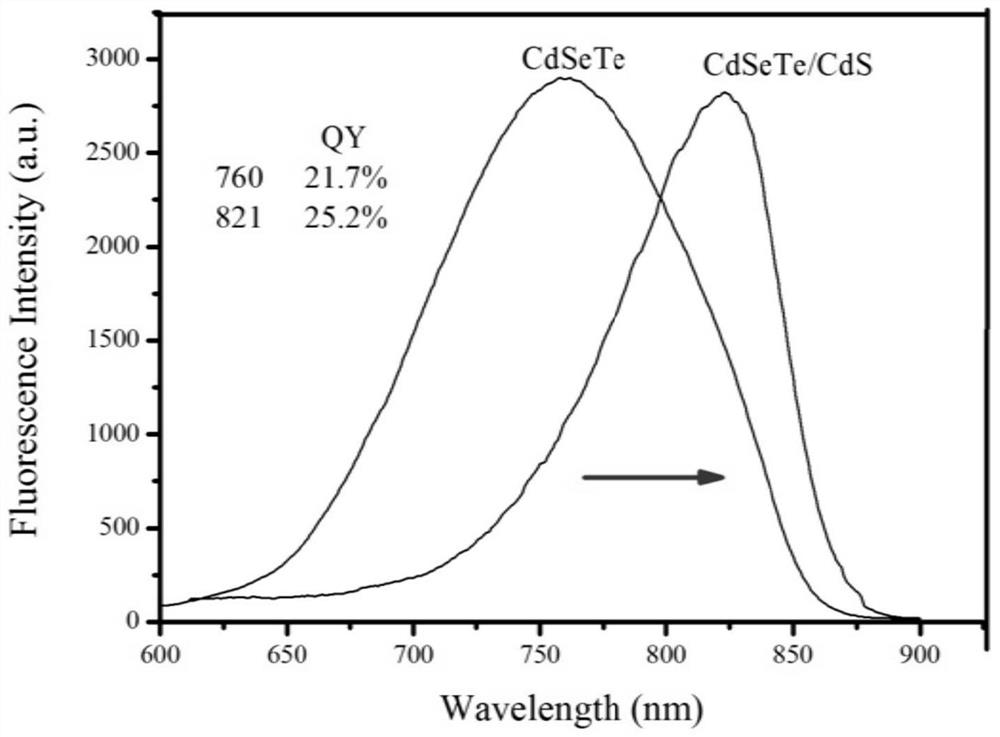
A Thermosensitive Liposome Released by Magnetothermal Release
A technology of heat-sensitive liposomes and liposomes, applied in liposome delivery, preparations for in vivo tests, medical preparations of non-active ingredients, etc., can solve the problems of poor precision of application methods and achieve selectivity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 101
[0091] DPPC is used as the basic material, and DSPG is used as the auxiliary material.
[0092] Add 750 mg of DPPC and 75 mg of DSPG to 20 mL of a mixed solution of chloroform and methanol (volume ratio of chloroform and methanol = 4:1), heat to 55 ° C, stir for 20 minutes until completely dissolved, and evaporate to dryness for 4 hours. The organic solvent is completely removed. Add 18 mL of phosphate buffer (pH=7.4±0.1), sonicate for 15 minutes, and then freeze-dry to obtain liposome microspheres.
[0093] Due to the limited tolerance of the human body to temperature, the basic material of TSL should be selected as a phospholipid with a lower phase transition temperature. Selected in this embodiment: DPPC, dipalmitoylphosphatidylcholine, phase transition temperature is 41 ℃. DSPG, distearoylphosphatidylglycerol, has a phase transition temperature of 55°C. DSPG is introduced because the drugs introduced later have little effect on the phase transition behavior of DSPG and ...
Embodiment 102
[0095] Thin film dispersion method
[0096] Weigh 500mg of DPPG, 200mg of DPSP, and 100mg of DSPC (55°C) in a rotary evaporator, add 30mL of chloroform, heat to 58°C, and evaporate completely on a rotary evaporator to remove chloroform. Forms a uniform film layer on the bottle wall. In addition, weigh 20mg RGD and add it into 15mL of phosphate buffer solution with pH=7.4, fully dissolve it, then add it into the aforementioned rotary steamer, mix well, stir for 10 minutes for hydration, and ultrasonic for 30 minutes to obtain milky white liposomes suspension. It is a heat-sensitive liposome material, which is freeze-dried to obtain a dry liposome material.
[0097] The selected DPPG (Tm=41°C), DPSP (Tm=41°C), DSPC (Tm=55°C), and several liposome raw materials are mixed together, and the phase transition temperature of the liposome shell after synergy is slightly higher than At 41°C, the stability of the encapsulated drug is higher, and the temperature of phase change relea...
Embodiment 103
[0099] freeze drying
[0100] Weigh 15 mg doxorubicin and dissolve it in an appropriate amount of phosphate buffer solution with pH=6.8, and sonicate for 15 minutes to obtain a doxorubicin solution. Then weigh a certain amount of DPPC 500mg and PSPC 500mg, add them to 30mL of anhydrous ether, stir magnetically for 10min, add the above-mentioned doxorubicin solution, homogenize at a high speed of 15000r / min for 5min, evaporate under reduced pressure at room temperature for 15 minutes, add 10mg of manna Alcohol, ultrasonic 20min, obtain emulsion, filter with 0.22 μm microporous membrane, filtrate freeze-dry with lyophilizer, obtain the liposome that wraps doxorubicin.
[0101] The selected PSPC (Tm=44°C), and the Tm of the liposomes prepared with DPPC are between 41 and 44°C, the phase transition temperature is suitable, and it can be used in a wide range of situations. The liposome obtained by freeze-drying is anhydrous dry liposome, and the liposome structure and function a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com