Alpha-galactosidase gene and application thereof
A galactosidase and gene technology, applied in the α-galactosidase gene and its application fields, can solve the problems of heterologous genes that are difficult to obtain high-efficiency expression, and achieve the effects of reducing complexity, increasing expression, and efficient degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] To construct a bacterial strain containing a single-copy vector of α-galactosidase, the method is as follows.
[0072] 1.1 According to the artificial design of the existing α-galactosidase gene (from Aspergillus niger) sequence, a new α-galactosidase gene sequence is designed, and the α-galactosidase gene sequence is obtained by artificial synthesis. - a galactosidase gene fragment whose base sequence is shown in SEQ ID NO: 1, named Gal A. The amino acid sequence of the alpha-galactosidase encoded by the alpha-galactosidase gene is shown in SEQ ID NO:2.
[0073] In order to increase the expression level of the α-galactosidase gene in Pichia pastoris, in this example, the sequence of the α-galactosidase gene was designed in silico with the assistance of software such as DNA 2.0. The following factors were mainly considered during the design process:
[0074] (1) Replace the original low-frequency codons with high-frequency codons in P. pastoris;
[0075] (2) reduced ...
Embodiment 2
[0086] Construction of recombinant expression vector with multiple copies of α-galactosidase gene
[0087] 2.1 The expression cassette fragment containing the α-galactosidase gene was obtained by double-enzyme digestion with the same tail enzyme, and the expression cassette fragment was connected to the single-copy expression vector of α-galactosidase to obtain a two-copy expression vector.
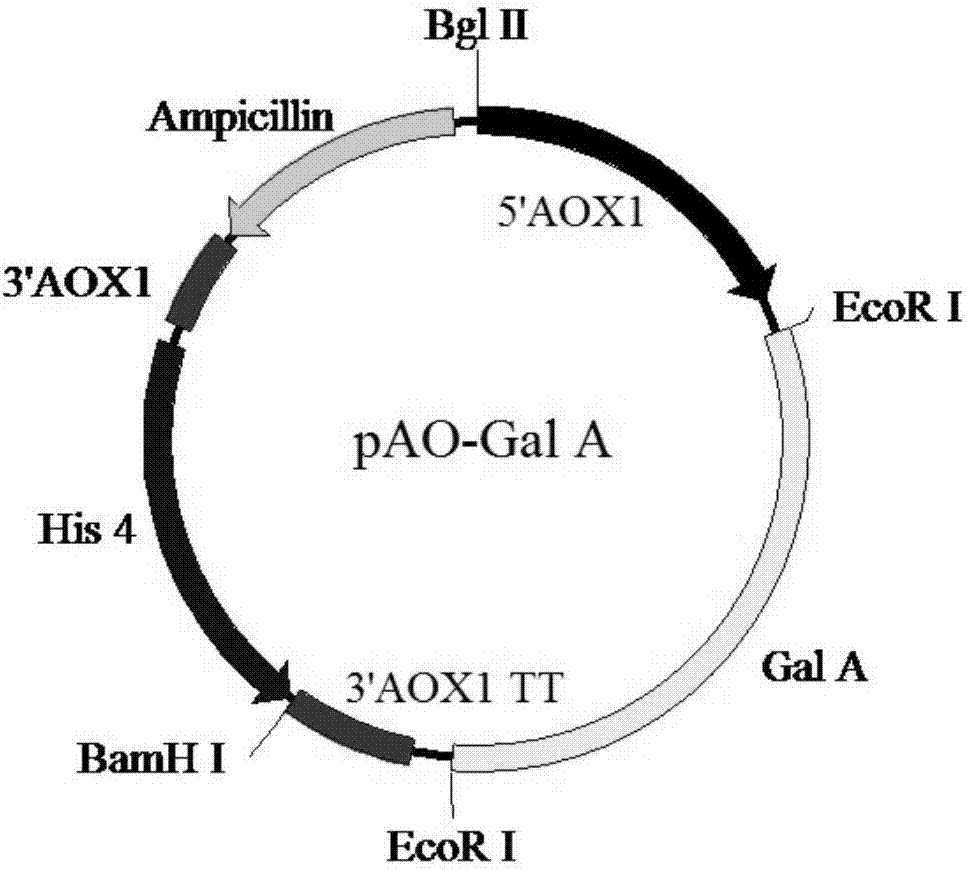
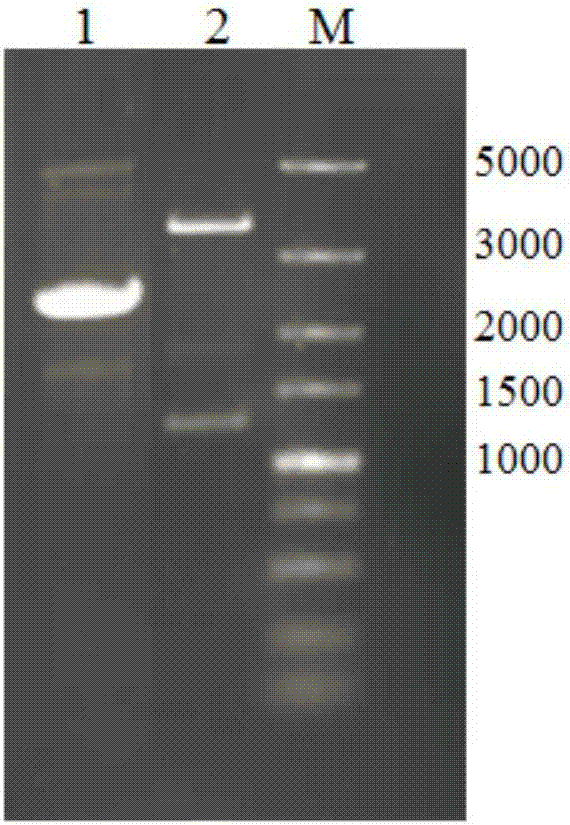
[0088] 2.1.1 After the pAO-Gal A recombinant expression vector obtained in Example 1 was digested with Bgl II and BamH I, the small fragment (ie, the AOX-Gal A expression cassette) was recovered from the gel, and the enzyme digestion system: 30 μL of pAO-Gal A expression vector, 1.5 μL of BamH I, 1.5 μL of Bgl II, 20 μL of 10×Buffer K, 20 μL of BSA, 20 μL of Triton X-100, ddH 2 Make up to 200 μL with O, and digest for about 4 hours at 37°C.
[0089] 2.1.2 Digest the new pAO-Gal A expression vector with BamH I and recover it from the gel, and connect it with the AOX-Gal A fragment obtained ...
Embodiment 3
[0102] Construction of co-expression vector of α-galactosidase gene and endoplasmic reticulum secreted protein-related genes
[0103] 3.1 Co-express genes related to endoplasmic reticulum secreted proteins such as HAC1, PDI, Ero1, Hsp40 and α-galactosidase gene respectively.
[0104] 3.1.1 HAC1, PDI, Ero1, and Hsp40 genes were respectively connected to the pAO815 vector to construct a recombinant vector of the endoplasmic reticulum secreted protein-related genes (the method is the same as step 1.2).
[0105] 3.1.2 Connect the expression cassette containing the α-galactosidase gene (the AOX-Gal A expression cassette obtained in step 2.1.1) to the recombinant vectors of the endoplasmic reticulum secreted protein-related genes obtained above to obtain Co-express the recombinant vector (for the method, refer to step 2.1). Wherein, the nucleotide sequence of the HAC1 gene is shown in SEQ ID NO: 3, the nucleotide sequence of the PDI gene is shown in SEQ ID NO: 4, the nucleotide seq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com