Screening system and application of REG Gamma-20S proteasome inhibitor
A technology of proteasome inhibitors and inhibitors, applied in the fields of biochemistry and biomaterials, to achieve the effects of high sensitivity, high specificity, and efficient detection capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
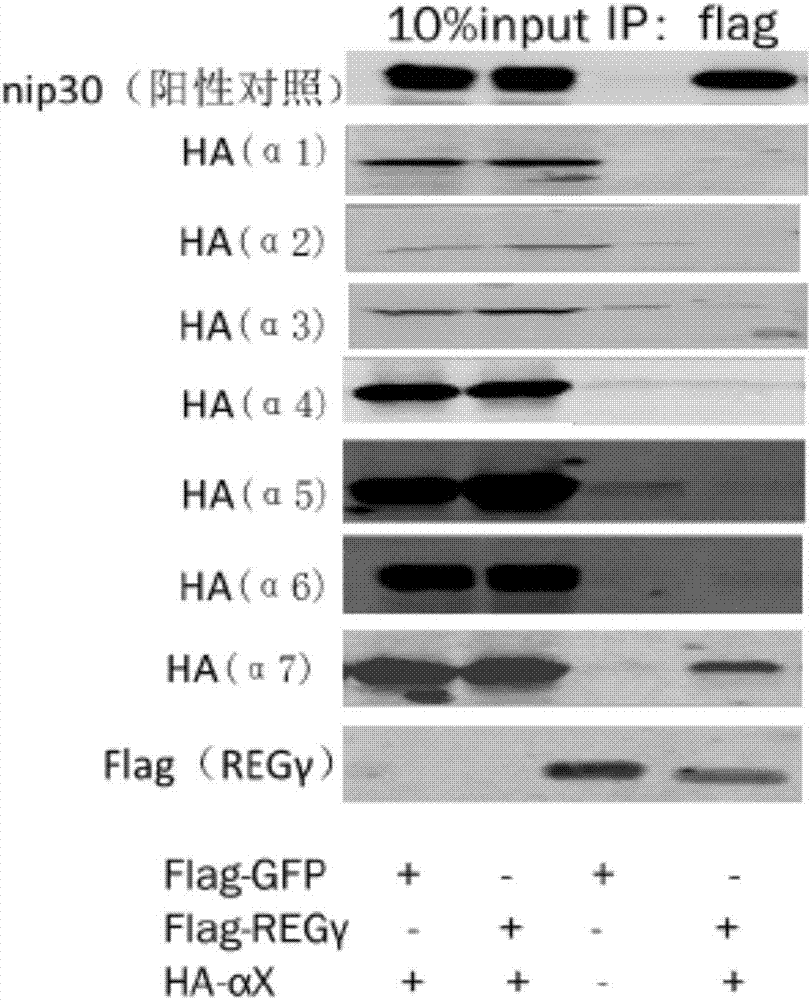
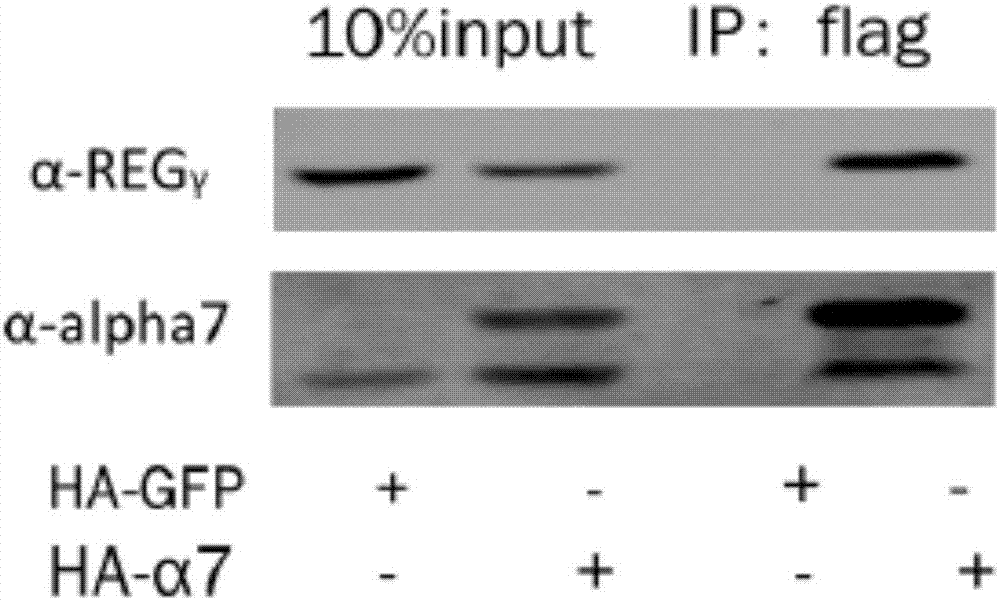
[0038] Example 1 Co-immunoprecipitation experiment proved that REGγ specifically binds to 20S proteasome α7 subunit
[0039] The α subunit of the 20S proteasome is its regulatory subunit, which is turned on or off by the regulation of activators. REGγ can promote the degradation of substrate proteins. It is likely that REGγ may open the 20S gate by binding to the α subunit. The present invention The interaction between REGγ and 20S proteasome α1-α7 was detected by immunoprecipitation method.
[0040] Specific experimental methods:
[0041] 1. Before transfection, plant HeLa cells in a six-well plate with a concentration of 60% and culture for 12 hours.
[0042] 2. Add 300μL of serum-free DMEM medium to a 1.5mL centrifuge tube, and then add the target plasmid and Young's transfection reagent. The ratio of the two is 1μg: 2μL. Each well of the six-well plate can transfer 1μg plasmid.
[0043] 3. Vortex and shake for 10 seconds to mix well. After standing at room temperature for 15 minute...
Embodiment 2
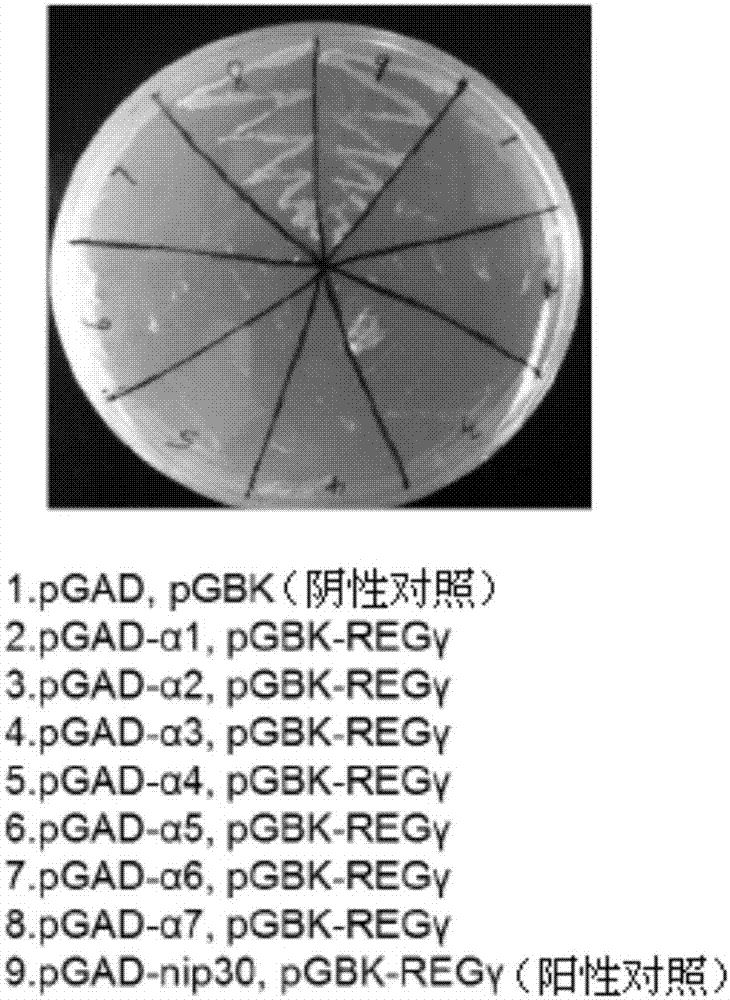
[0052] Example 2 Yeast two-hybrid experiment proved that REGγ specifically binds to 20S proteasome α7 subunit
[0053] Different experiments proved that REGγ specifically binds to the α7 subunit of the 20S proteasome.
[0054] In the experiment, PSMA1-PSMA7 (α 1 -α 7 ) Was constructed on pGADT7 vector, and tried to use yeast two-hybrid experiment to further verify the interaction between REGγ and α7.
[0055] Yeast two-hybrid results such as image 3 As shown, only when PGAD-α7 / pGBK-REGγ cotransforms, yeast can grow on the four-deficiency plate, which means that REGγ only interacts with the α7 subunit, which is consistent with the result of immunoprecipitation ( nip30 as a positive control).
[0056] Specific experimental methods of yeast two-hybrid experiment:
[0057] 1. Take out the yeast strain from the refrigerator at -80℃ and melt it at room temperature, then heat the inoculation loop to red hot on an alcohol lamp. After cooling, use the inoculation loop to dip a small amount of ...
Embodiment 3
[0068] Example 3 Construction of a screening system for inhibitors of the interaction between REGγ and α7 using luciferase fragment complementation technology
[0069] By comparing several commonly used molecular imaging techniques for studying protein-protein interactions, such as energy resonance transfer technology, green fluorescent protein fragment complementation technology, and luciferase fragment complementation technology. The present invention finally selects the efficient and convenient luciferase fragment complementation technology as the system basis for screening inhibitors of the interaction between REGγ and α7. In the design experiment, the present invention considers three key issues: The first key issue is (1) the cleavage site of luciferase, how to cut the luciferase so that the two fragments produced by it can recombine well And the newly bound luciferase can regain the activity of luciferase. Such as Figure 4 As shown, the present invention selects the most...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com