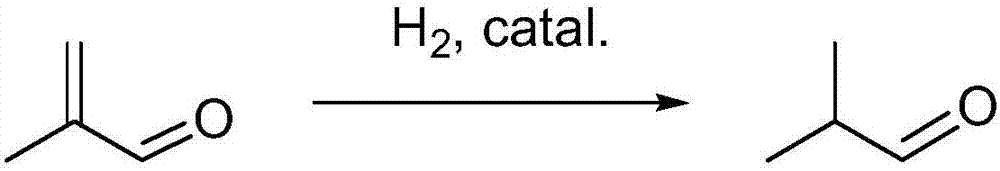
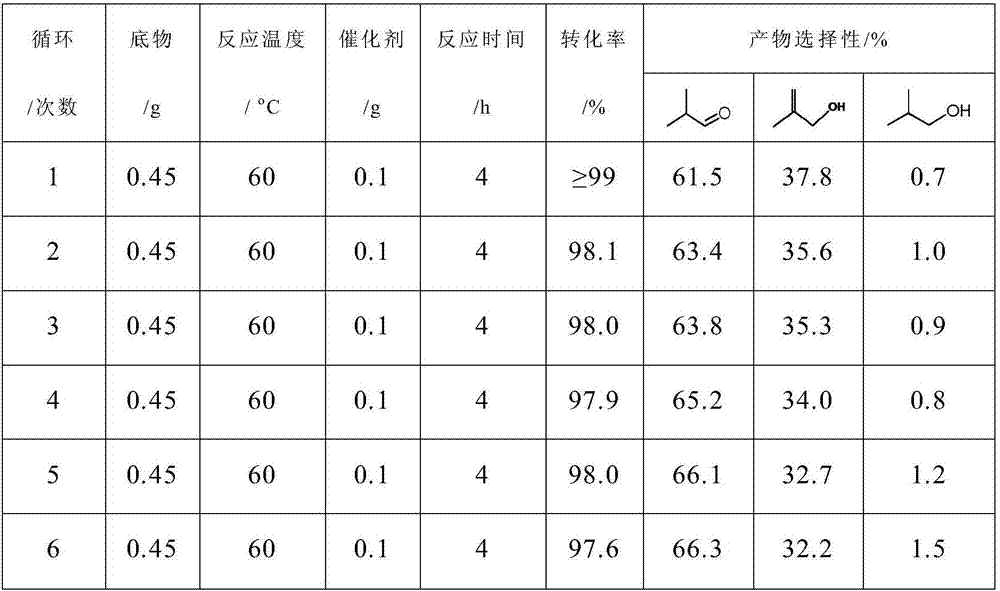
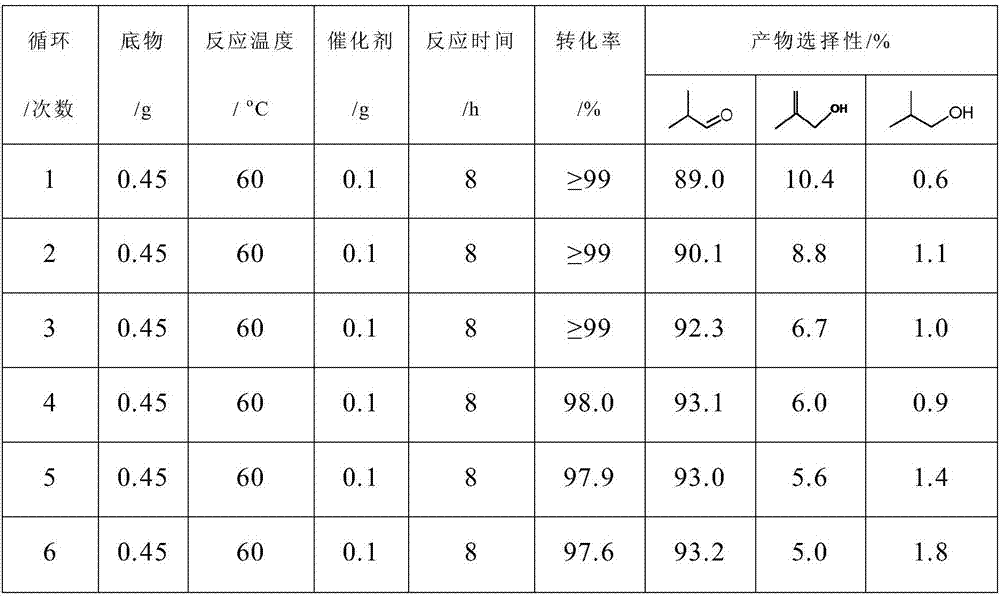
Selective hydrogenation catalyst, preparation method and catalyzing evaluation method on generation of isobutyraldehyde by the selective hydrogenation catalyst
A technology of hydrogenation catalyst and evaluation method, which is applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalyst, carbon-based compound preparation, etc. , the effect of mild reaction conditions, low temperature and pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] A kind of preparation method of above-mentioned selective hydrogenation catalyst, it comprises the steps:
[0032] Step 1: Weigh a part of the carrier in a beaker, weigh another part of the carrier as a carrier for impregnation, and pour deionized water into the beaker under stirring conditions until the carrier in the beaker is just saturated, and record the consumption of deionized The volume of water obtains the water absorption of the unit mass carrier, i.e. the saturated water absorption W of the carrier is in mL / g;
[0033] Step 2: Dissolve the noble metal precursor in water, dissolve under heating conditions, and mark it as solution A;
[0034] Step 3: Dissolve the non-noble metal precursor in water, marked as solution B;
[0035] Step 4: Mix solution A and solution B evenly, dilute and set aside, and mark it as impregnating solution C. The volume of impregnating solution C is equal to the weight of the impregnating carrier multiplied by the saturated water abso...
Embodiment 1
[0054] Selective Hydrogenation Catalyst 4% Ir-MoO 3 / SiO 2 (0.9) is prepared as:
[0055] Step 1: Put 0.286g of purchased precipitated silica in a beaker, drop in deionized water until the carrier is saturated, record the volume of consumed water as 2.6mL, that is, the saturated water absorption W of the carrier is 9.1mL / g;
[0056] Step 2: Take 0.022g iridium trichloride hydrate, dissolve it in 1.0mL water, heat at 50°C for 0.5 hour to form a transparent red solution, which is marked as solution A;
[0057] Step 3: Take 0.01g of ammonium paramolybdate, dissolve it in 0.5mL of water, and mark it as solution B;
[0058] Step 4: Mix solution A and solution B evenly to form an impregnating solution, add water to dilute to 2.6mL, and mark it as impregnating solution C. The molar concentration of noble metal atoms in impregnating solution C is 0.024mol / L, and the molar concentration of non-noble metal atoms is 0.022 mol / L;
[0059] Step 5: Take another 0.286g of precipitated si...
Embodiment 2
[0062] Selective Hydrogenation Catalyst 4% Ir-MoO 3 / SiO 2 (2) is prepared as:
[0063] Step 1: Put 0.286g of purchased precipitated silica in a beaker, drop in deionized water until the carrier is just absorbed and saturated, record the volume of consumed water as 2.6mL, that is, the saturated water absorption W of the carrier is 9.1mL / g ;
[0064] Step 2: Take 0.022g iridium trichloride hydrate, dissolve it in 1.0mL water, heat at 50°C for 0.5 hour to form a transparent red solution, which is marked as solution A;
[0065] Step 3: Take 0.022g of ammonium paramolybdate, dissolve it in 0.5mL of water, and mark it as solution B;
[0066] Step 4: Mix solution A and solution B evenly, add water to dilute to 2.6mL, and form an impregnation solution, which is marked as impregnation solution C. The molar concentration of noble metal atoms in impregnation solution C is 0.024mol / L, and the molar concentration of non-noble metal atoms is 0.048mol / L;
[0067] Step 5: Take another 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Saturated water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com