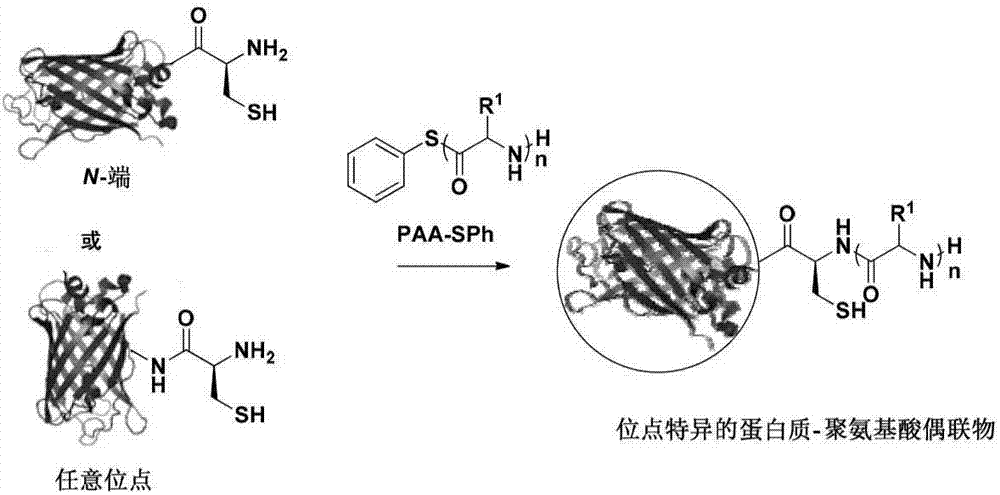
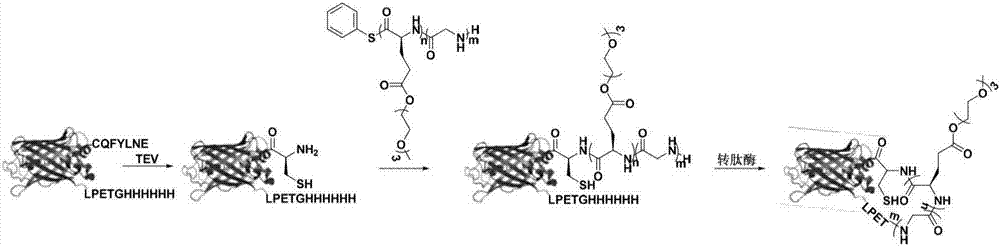
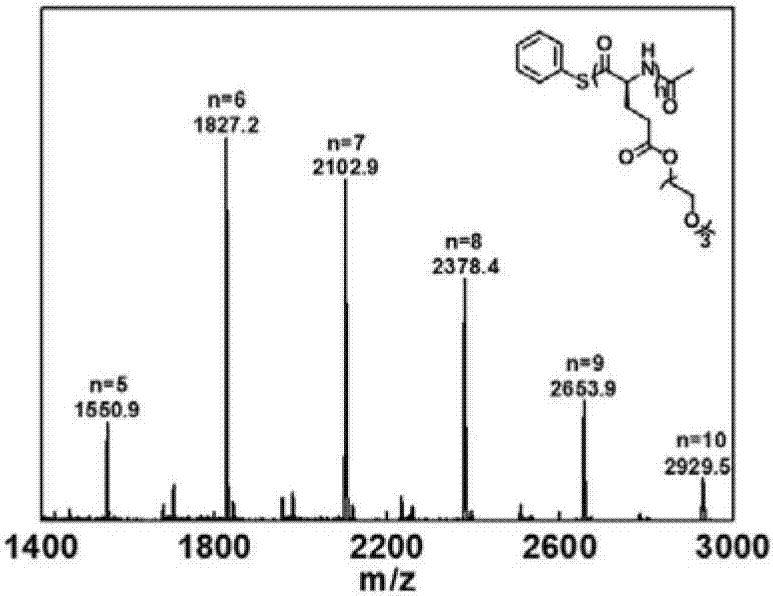
Method for preparing protein-polyamino acid cyclic conjugate
A polyamino acid and protein technology, applied in the field of biomedicine, can solve problems such as accelerated clearance, low immunogenicity, and accumulated toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177] Example 1: Synthesis of polyamino acid P1 for protein coupling
[0178]
[0179] L-glutamic acid (ethylene glycol) in the glove box 3 Ester-N-carboxylide anhydride (N1, 40.0 mg, 0.131 mmol, 20 equiv) was dissolved in anhydrous N,N-dimethylformamide (1.0 mL) and added trimethylsilylthiophenol in DMF Solution (13.6μL×0.5M, 1.0 equivalent), stirred at room temperature for 25 hours, then poured the reaction solution into 40mL ether solution, and the white precipitate was the product. Centrifuge to obtain a solid product, pour out the supernatant and rinse the product with 40 mL of ether again, pour the supernatant after centrifugation to obtain a solid and dry it in a vacuum oven to obtain 30 mg of a colorless transparent colloidal solid (yield 72%), which is stored in - 20 ℃ refrigerator.
[0180] Poly-L-glutamic acid (ethylene glycol) with an average degree of polymerization of 7 was synthesized in a manner similar to the above method 3 Esters, simply add L-glutam...
Embodiment 2
[0181] Example 2: Synthesis of block polyamino acid P2 for protein cyclization
[0182]
[0183] L-glutamic acid (ethylene glycol) in the glove box 3Ester-N-carboxylide anhydride (N1, 13.0 mg, 0.044 mmol, 7 equiv) was dissolved in anhydrous N,N-dimethylformamide (1.0 mL) and trimethylsilylthiophenol in DMF was added Solution (13.1μL×0.5M, 1.0 equivalent), stirred at room temperature for 25 hours, then added glycine-N-carboxy internal anhydride (N2, 2.0mg, 0.0197mmol, 3 equivalents), continued to stir at room temperature for 2 hours, post-treatment method Same as Example 1.
Embodiment 3
[0184] Example 3: Synthesis of polyamino acid P4 for protein coupling
[0185]
[0186] Ethyl phosphate-L-tyrosine N-carboxylidene anhydride (N3, 100 mg, 0.291 mmol, 20 equiv) was dissolved in anhydrous N,N-dimethylformamide (2.0 mL) in a glove box, and A DMF solution of trimethylsilylthiophenol (29.1 μL×0.5M, 1.0 equivalent) was added, stirred at room temperature for 25 hours, and the post-treatment method was the same as in Example 1.
[0187] The above polyamino acid (P3, 80mg, 0.0133mmol, 1.0eq) was dissolved in dichloromethane (1.5mL), and triethylamine (300μL, 2.1mmol) and trimethylbromosilane (360μL, 2.7mmol) were added sequentially . The reaction solution was heated to 60°C and stirred for 8 hours. After the solvent was spin-dried on a rotary evaporator, the product was dissolved in deionized water (4mL), and EDTA (10mg) was added, and the pH was adjusted with aqueous sodium hydroxide solution (1M). to neutral, and dialyzed against aqueous sodium chloride (1000D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com