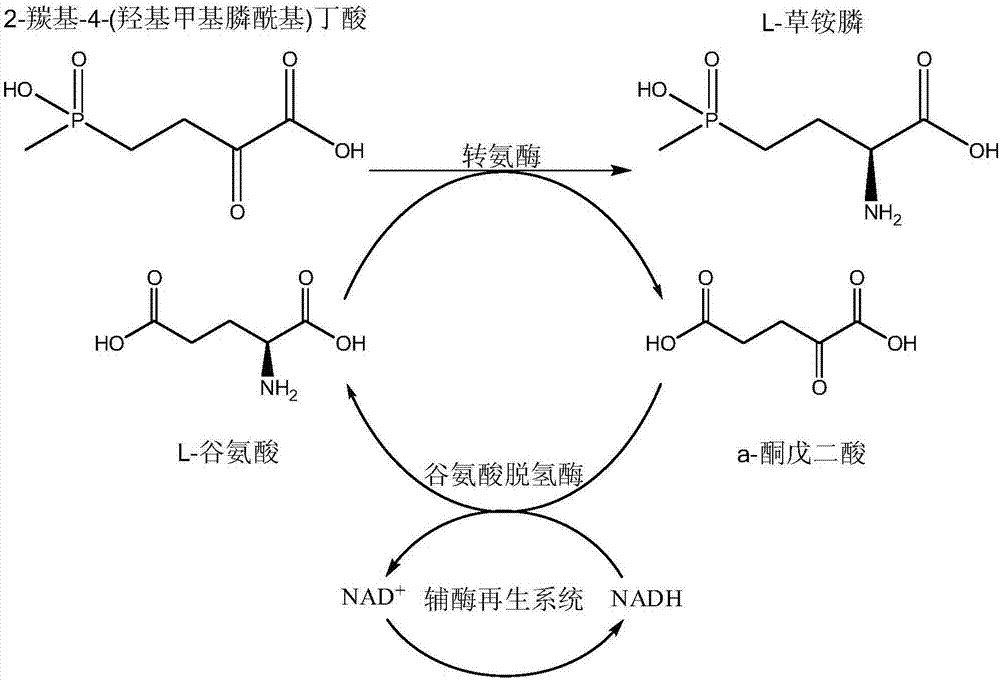
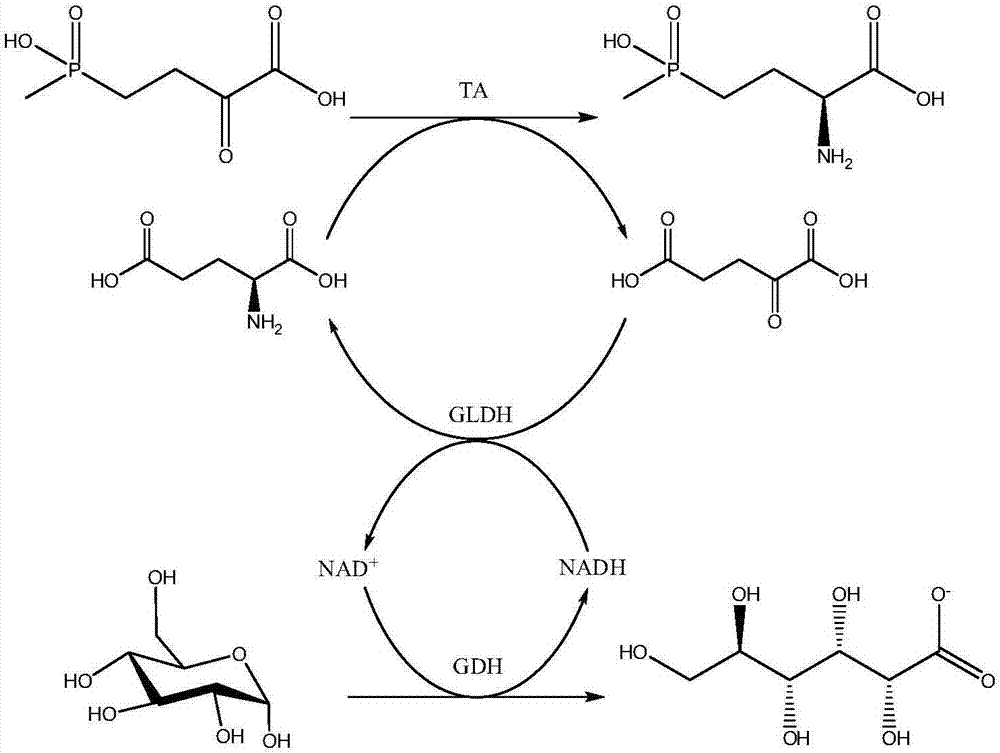
Production method of L-phosphinothricin
A technology for the regeneration of glufosinate-ammonium and coenzyme, applied in the biological field, can solve the problems of only 52% reaction conversion rate, little advantage compared with chemical synthesis, low optical purity of products, etc. Overall yield and strong stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 Genetic Engineering Bacteria Strain Construction
[0058] 1.1 Construction of genetically engineered bacteria expressing γ-aminobutyric acid / α-ketoglutarate aminotransferase
[0059] The transaminase genes were cloned from the genomes of Escherichia coli E.coli K12W3110, Bacillus subtilis 168 and Bacillus magaterium YYBM1 respectively. ) to design corresponding PCR upstream primers and downstream primers.
[0060] Primers for transaminases derived from E.coli:
[0061] EC-F sequence: 5'-CCG GAATTC ATGAGCAACAATGAATTCCATC-3' (EcoRI)
[0062] EC-R sequence: 5'-CCG CTCGAG TTAATCGCTCAGCGCATCC-3'(XholI)
[0063] Primers for transaminases from Bacillus subtilis:
[0064] BS-F sequence: 5'-CCC GAGCTC ATGAGTCAAAACAACAGCAAGCATCA-3'(SacI)
[0065] BS-R sequence: 5'-CCC AAGCTT TTAAGCTCGCAGGCCCGCCT-3' (HindIII)
[0066] Primers for transaminases from Bacillus magaterium:
[0067] BM-F sequence: 5'-CGC GGATCC ATGAGTCAAACTTTTAGCAA-3' (BamHI)
[0068] BM-...
Embodiment 2
[0105] 2.1 Culture of microorganisms
[0106] Composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 121°C for 20min, ready for use.
[0107] The genetically engineered bacteria E.coli BL21(DE3) containing the transaminase gene was inoculated into 5 mL LB liquid medium containing 50 μg / mL kanamycin, and cultured with shaking at 37°C for 12 hours. Transfer to 500mL fresh LB liquid medium also containing 50μg / mL Kan, shake culture at 37°C until OD 600 When it reaches about 0.8, add IPTG to its concentration of 0.3mM, and induce culture at 28°C for 20h. After the cultivation, the culture solution was centrifuged at 10,000 rpm for 10 min, the supernatant was discarded, and the bacterial cells were collected, and stored in a -70°C ultra-low temperature refrigerator until use.
[0108] 2.2 Preparation of crude enzyme solution
[0109] The bacterial cells collected after the cultivation were...
Embodiment 3
[0119] γ-aminobutyric acid / α-ketoglutarate aminotransferase derived from Escherichia coli K12W3110, glutamate dehydrogenase derived from Escherichia coli K12W3110, glucose dehydrogenase derived from Bacillus megaterium DSM319, Refer to Example 1 for strain construction. The cells were collected after microbial culture, and the enzyme activity of the wet cells was measured. The enzyme activity assay method refers to Example 2. The enzyme activities of the three strains of cells are 932.5U / g wet cells, 338.3U / g wet cells, and 993.3U / g respectively. wet cell
[0120] A solution containing 100 mM of PPO, 5 mM of glutamic acid, 1 mM of pyridoxal phosphate, 120 mM of glucose and 0.1 mM of NADH was placed in a warm bath at 37°C, and the pH of the solution was adjusted to 7.5 with 30% ammonia water. Add 5 g / L of engineering bacterial cells expressing γ-aminobutyric acid / α-ketoglutarate aminotransferase derived from Escherichia coli E.coli K12W3110, and engineering bacteria expressing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pre-denatured | aaaaa | aaaaa |
| Extend | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com