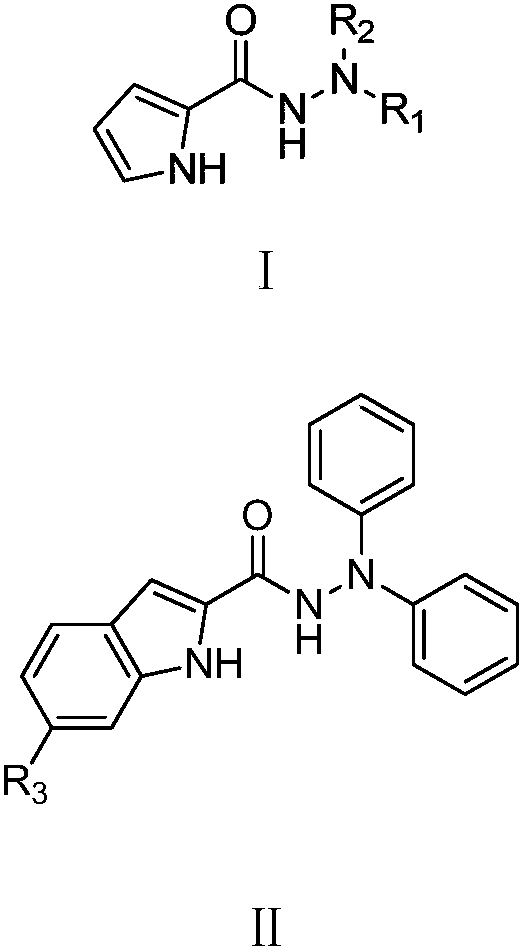
A copper-catalyzed c-n coupling method using n,n-disubstituted hydrazides as ligands
A two-substitution, copper-catalyzed technology, applied in the chemical field, can solve the problems of narrow substrate application range and high reaction temperature, and achieve the effects of wide application range, simple operation and high chemoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
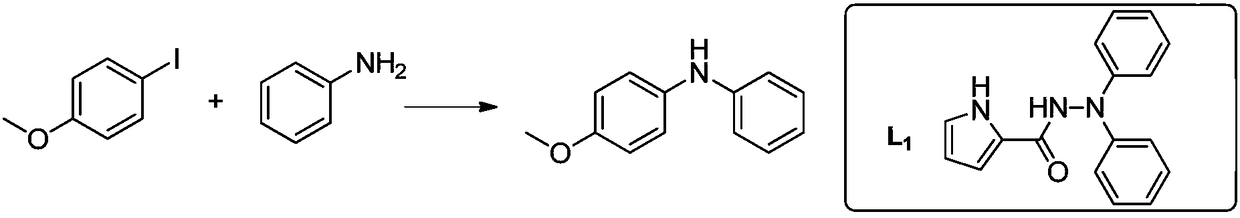
[0026] Embodiment 1: the synthesis of N-p-methoxyphenylaniline
[0027]
[0028] With 234mg (1mmol) 4-iodoanisole, 93mg (1mmol) aniline, 25mg (0.1mmol) CuSO 4 ·5H 2 O, 14 mg (0.1 mmol) Ligand L1, 138 mg (1 mmol) K 2 CO 3 , 2mL EtOH, added to a 10mL reaction tube, sealed, and reacted at 20°C for 48h. After the reaction stopped, add water, extract with ethyl acetate, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, distill the filtrate under reduced pressure, and separate and purify by silica gel column chromatography to obtain N-p-methoxyphenyl Aniline 181 mg, yield 91%.
[0029] MS (ESI + ): m / z: 200 ([M+H] + ); 1 H NMR (400MHz, CDCl 3 )δ7.27-7.23(m,2H,ArH),7.15-7.07(m,2H,ArH),6.97-6.95(m,2H,ArH),6.89(d,J=8.7Hz,3H,ArH), 3.83(s,3H,OCH 3 ).
[0030] Ligand: MS (ESI + ): m / z: 300 ([M+Na] + );1H NMR(400MHz,DMSO)δ11.63(s,1H,NH),10.76(s,1H,NH),7.31-7.28(m,4H,ArH),7.16-7.14(m,4H,ArH) , 7.00-6.98 (m, 4H, ArH), 6.16 (br, 1H, ArH).
Embodiment 2
[0031] Embodiment 2: the synthesis of N-benzylaniline
[0032]
[0033] With 156mg (1mmol) bromobenzene, 139mg (1.3mmol) benzylamine, 9.5mg (0.05mmol) Cu1, 10.8mg (0.05mmol) ligand L2, 276mg (2mmol) K 2 CO 3 , 2mL of diethylene glycol (DEG), was added into a 10mL reaction tube, sealed, and reacted at 25°C for 72h. After the reaction stopped, add water, extract with ethyl acetate, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, distill the filtrate under reduced pressure, and separate and purify by silica gel column chromatography to obtain 156 mg of N-phenylbenzylamine. Yield 85%.
[0034] MS (ESI + ): m / z: 184 ([M+H] + ); 1 H NMR (400MHz, CDCl 3 )δ7.45–7.35(m,5H,ArH),7.26(t,J=7.5Hz,2H,ArH),6.81(t,J=7.1Hz,1H,ArH),6.72(d,J=7.8Hz ,2H,ArH),4.40(s,2H,NCH2).
[0035] Ligand: MS (ESI + ): m / z: 238 ([M+Na] + ); 1 H NMR (400MHz, DMSO) δ11.57(s, 1H, NH), 10.18(s, 1H, NH), 7.20(t, J=7.7Hz, 2H, ArH), 6.94-6.92(m, 2H, ArH ),6.80–6.73(m...
Embodiment 3
[0039] Example 3: 2-methoxy-N-phenylaniline
[0040]
[0041] 186mg (1mmol) 2-bromoanisole, 186mg (2mmol) aniline, 9.05mg (0.05mmol) Cu (OAc) 2 , 36.1 mg (0.1 mmol) ligand L3, 489 mg (1.5 mmol) Cs 2 CO 3 , 2mL MeOH, added to a 10mL reaction tube, sealed, and reacted at 60°C for 3h. After the reaction stopped, add water, extract with ethyl acetate, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, distill the filtrate under reduced pressure, and separate and purify by silica gel column chromatography to obtain 2-methoxy-N- 175mg of phenylaniline, yield 88%. MS (ESI + ): m / z: 200 ([M+H] + ); 1 H NMR (400MHz, CDCl 3 )δ7.37–7.28(m,3H,ArH),7.21(d,J=8.1Hz,2H,ArH),7.01–6.92(m,4H,ArH),3.93(s,3H).
[0042] Ligand: MS (ESI + ): m / z: 362 ([M+H] + ); 1 H NMR (400MHz,DMSO)δ11.40(s,1H,NH),10.45(s,1H,NH),7.30–7.28(m,2H,ArH),7.11–7.03(m,6H,ArH),6.90 -6.86(m,2H,ArH),6.08(d,J=2.1Hz,1H,ArH),3.45-3.40(m,2H,CH),1.03(d,12H,CH 3 );
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com