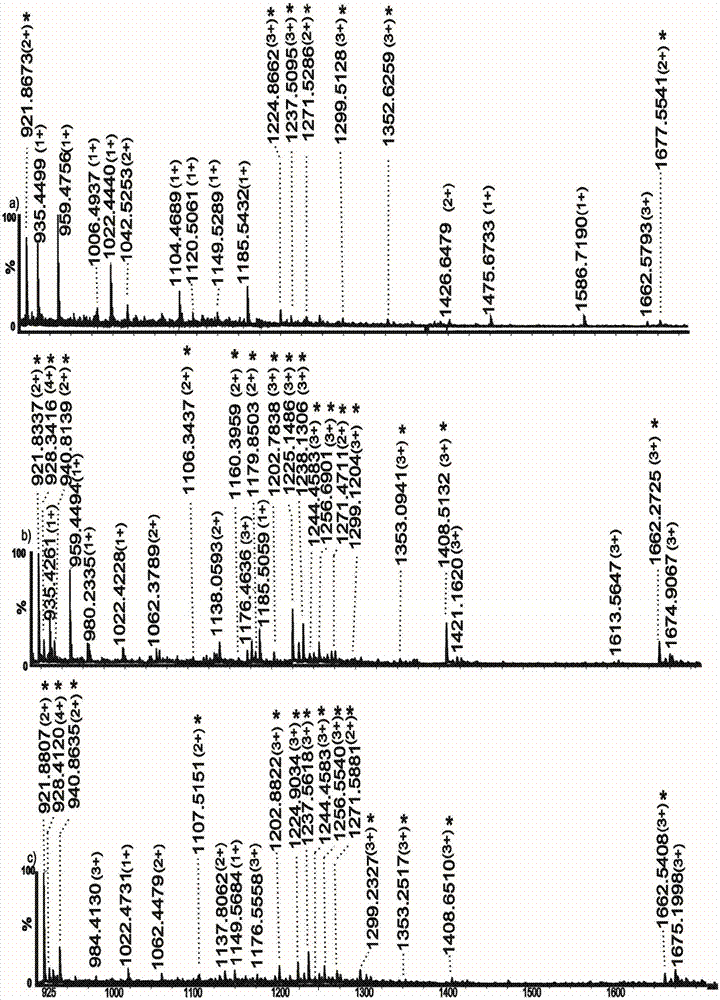
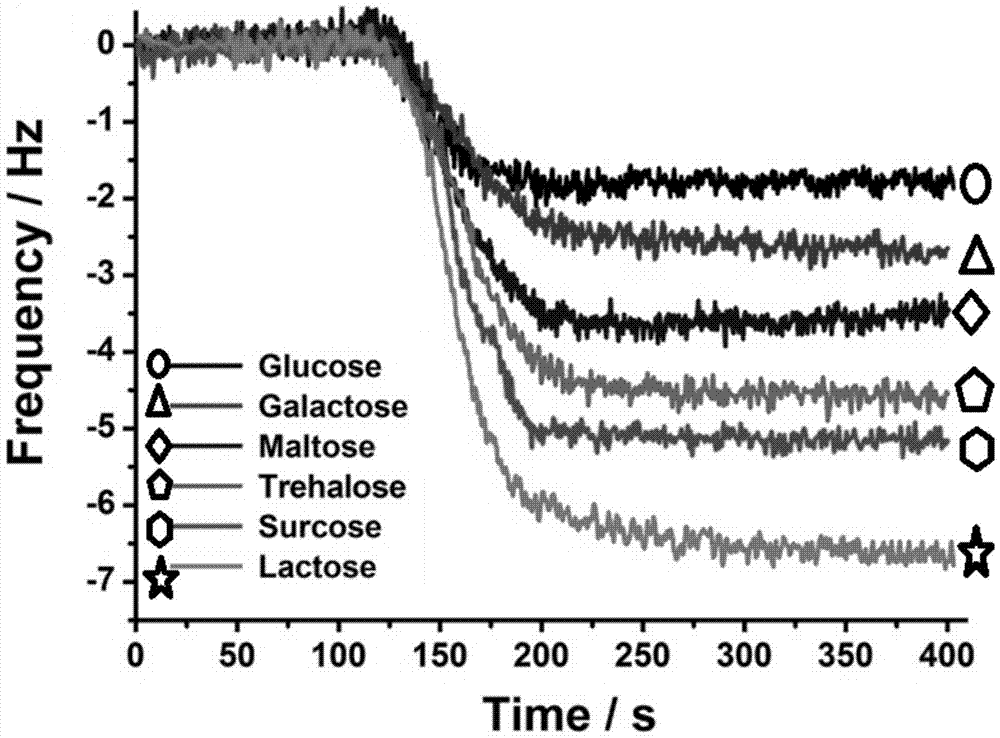
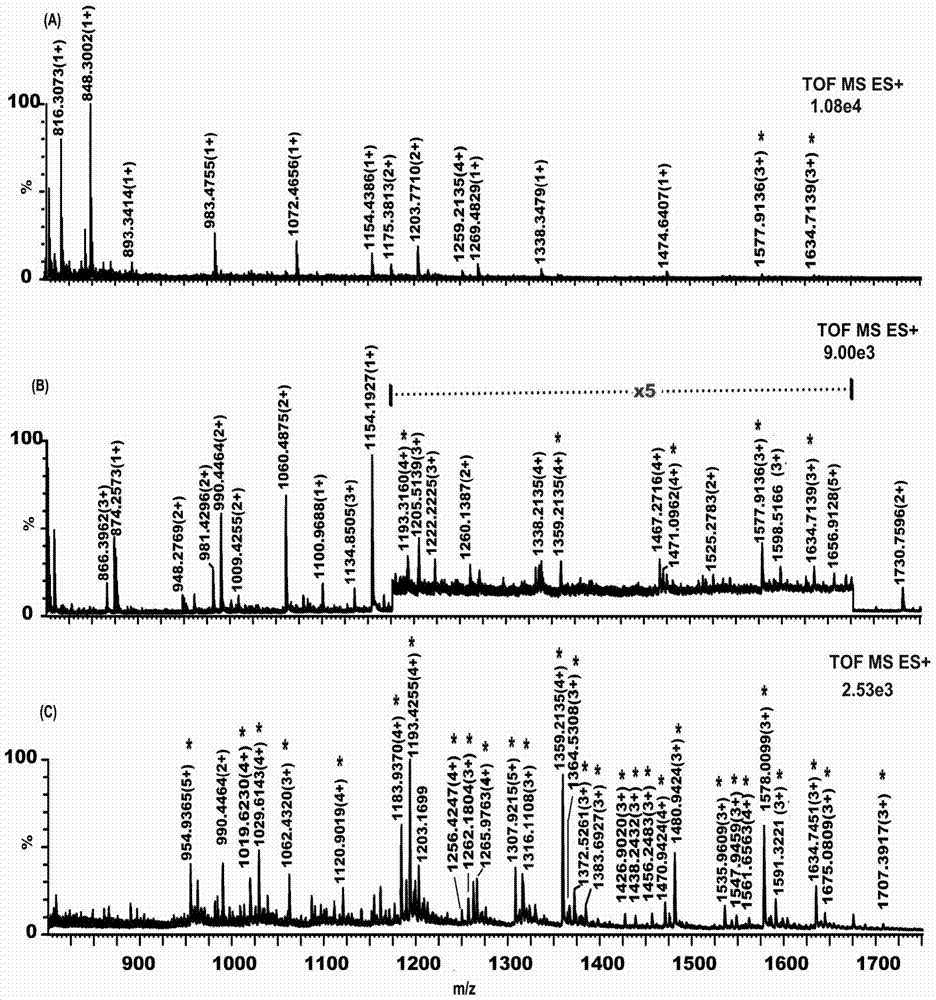
Monosaccharide polymer enriching material and preparation thereof, and application of the monosaccharide polymer enriching material to enrichment of glycopeptides
A polymer and monosaccharide technology, which is applied in analytical materials, preparation methods of peptides, preparation of samples for testing, etc., and can solve the problems of inability to achieve glycoproteomics, low glycosylation coverage, and damage to sugar chains.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Synthesis of Glucose Functional Monomer
[0067] Add 200ml of acetic anhydride solution into the reaction vessel, add 1ml of perchloric acid, slowly add 20g of required glucose, stir for 30min, put it in an ice bath, add 6g of red phosphorus, 20ml of bromine, and 14ml of water in turn, and then stir for 4h at room temperature . The resulting mixture was filtered, dried, and then washed three times with 50% petroleum ether ether solution, and spin-dried to obtain a glucose derivative in which other hydroxyl groups were brominated at the first position and protected by acetyl groups;
[0068] Add 20g of the above product into the reaction vessel, dissolve it with 150ml of chloroform, then add 150ml of saturated sodium carbonate solution, add 5g of tetrabutylammonium iodide, 8g of p-hydroxybenzaldehyde, react at 60 degrees Celsius for 12h, then remove the water layer, After drying, the mixture is purified by column chromatography. The first position is connected with p-hy...
Embodiment 2
[0075] Preparation of glycopolymer films
[0076]
[0077] In sugar polymers, x:0.01~0.5
[0078] Taking x=0.2 as an example, add 2g polyacrylamide and 0.5g monosaccharide functional monomer to a 50mL flask successively, add 10mL H2O and 15mL methanol as solvent at the same time, stir at 60°C for 48 hours, and the obtained product is mixed with methanol and After three days of dialysis in a mixed solution of water, the monosaccharide polymer was obtained as a white solid after freeze-drying.
[0079] Glycopolymer Characterization Data 1H NMR(500MHz, D2O): δ(ppm): 1.62(m, 20H, C-CH2), 2.20(d, 10H, C-CH), 3.39-3.96(m, 5H, CH-OH and CH2-OH) ,4.21(t,J=3.0Hz,1H,CH-OH),5.43(d,J=8.1Hz,1H,O-CH-O),7.21(m,2H,Ph-H),7.89(d, J=7.1Hz, 2H, Ph-H), 9.75(s, 1H, CH=N).IR(cm-1): 3346, 3202, 2927, 1658, 1606, 1579, 1510, 1452, 1429, 1399, 1350,1322,1257,1172,1115,1102,1072,1036,912,838,821,804,701,611.
[0080] Si, SiO2, Au, Ag, Pt, CuO, Al2O3, TiO2, ZrO2 or Fe3O4 connected with isothiocya...
Embodiment 3
[0082] Preparation of Glycopolymer Silica Functional Surface
[0083] Immerse the porous silica gel with isothiocyanate groups in the aqueous solution containing the above-mentioned monosaccharide aggregate; control the temperature of the flask at 60°C and let it stand for 4-6 hours; after the reaction, wash the polymer graft with methanol and H2O in sequence Porous silica gel was dried under vacuum at 30°C and placed in a desiccator for later use. The same method can be used to prepare silica gel samples grafted with smart glycopolymers with different particle sizes (including silica gel particle size and pore size), which can be used as packing materials for glycopeptide enrichment and separation columns. Figure 4 It is a picture of thermogravimetric analysis, a comparison chart of silica gel connected with sugar smart polymer and silica gel connected with isothiocyanate group;
[0084]
[0085] Structure and Synthetic Method of Functional Monomer
[0086] In order to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com