The gene encoding the biosynthetic enzyme of glucodipeptide and its application
A kind of dipeptide and biosynthetic technology applied in the biological field, which can solve the problems of inability to be widely used in the market, high cost of enzyme separation and purification, unsuitable for industrial production, etc., and achieve obvious market competitiveness, fast reaction rate, equipment operation and control easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
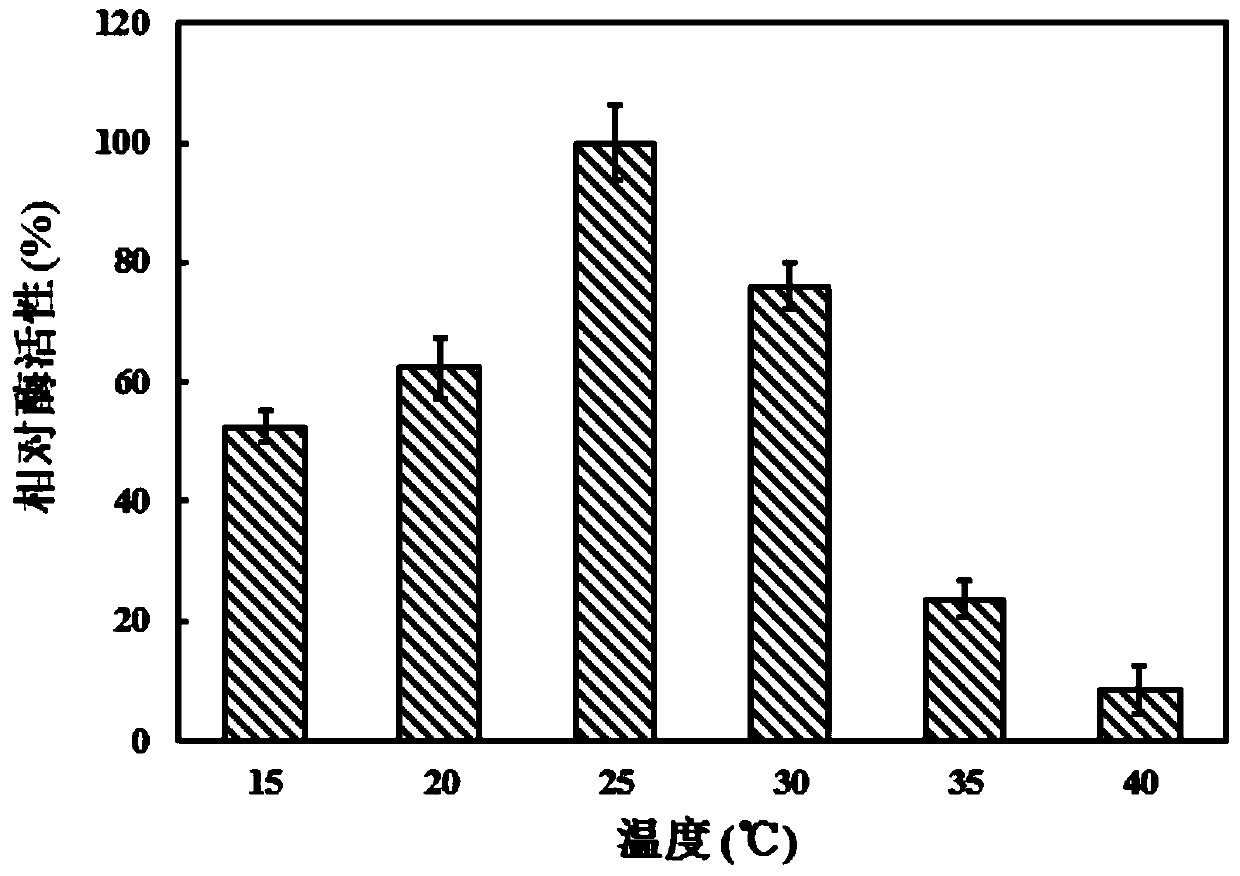
Examples
preparation example Construction
[0025] Next, the present invention provides a biosynthetic method based on the above-mentioned recombinant Escherichia coli to synthesize dipeptide. The method at least includes the process of fermenting and producing the recombinant Escherichia coli obtained by the above method according to the prior art, which involves the step of transformation reaction of the recombinant Escherichia coli to the substrate.
[0026] The biosynthesis of CG dipeptide is a relatively mature technology, and its substrate can be described as a substrate comprising a carboxyl component and an amine component according to the prior art. In the present invention, the carboxyl component is selected from amino acid esters and amino acid Amide, most preferably L-alanine methyl ester hydrochloride; said amine component is selected from amino acids, C-protected amino acids and amines, most preferably L-glutamine. The substrate concentration in the reaction system is set according to the proportional reac...
Embodiment 1
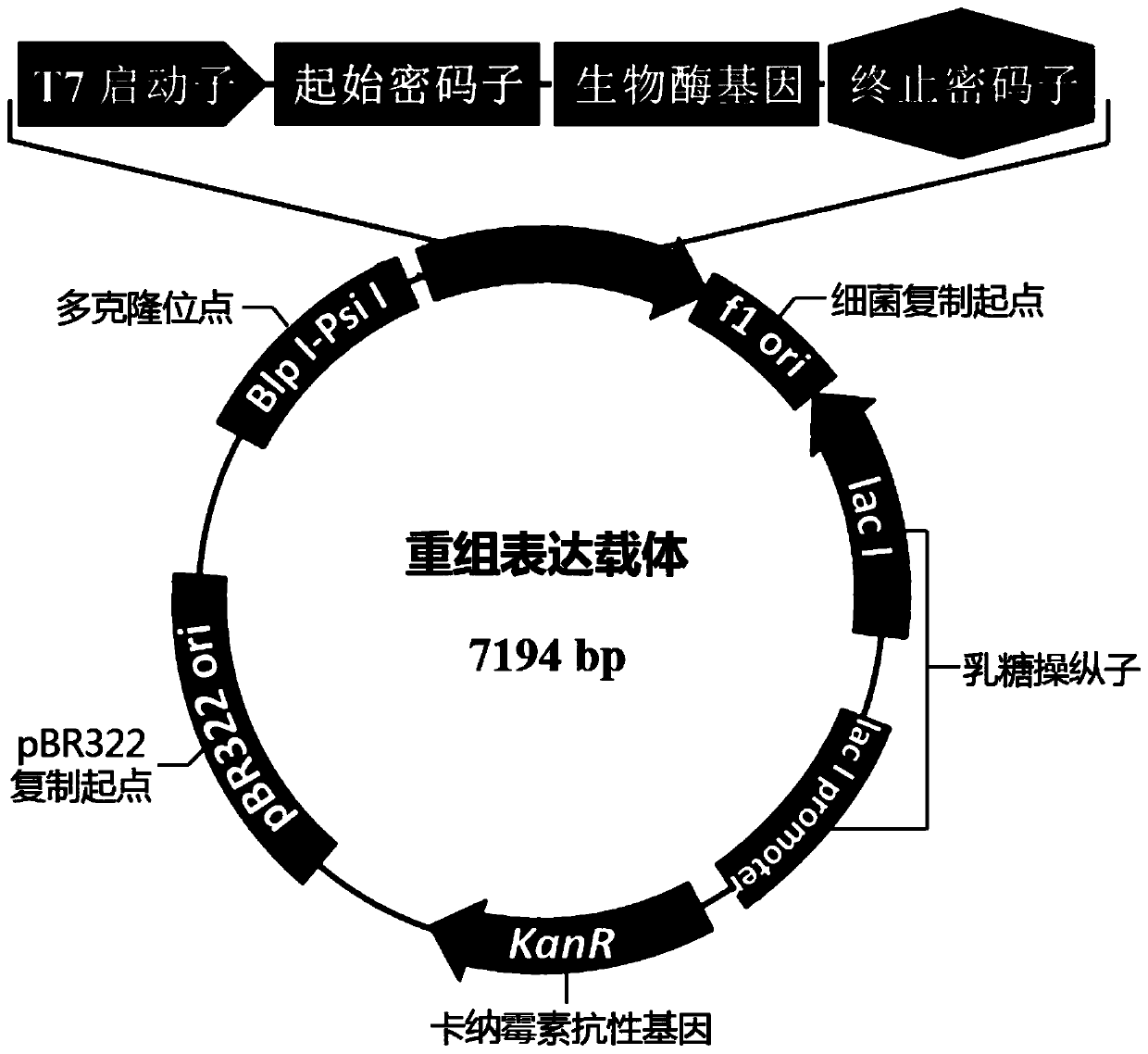
[0048] Construction of biological enzyme expression vector and recombinant Escherichia coli:
[0049] According to the known nucleotide sequence (SEQ ID NO.5) of related biological enzymes, primers (SEQ ID NO.3 / 4) were designed to derive from Sphingobacter siyangii ( Sphingobacterium siyangensis, strain number: 1.6855) genome is used as a template to amplify the biological enzyme gene SEQ ID NO.1.
[0050] Among them, the PCR reaction system (50μL):
[0051]
[0052] PCR reaction conditions:
[0053]
[0054] After the PCR product was recovered and purified by gel, it was combined with the plasmid pMD TM 19-T connection, positive clones were sequenced after extracting plasmids. The bioenzyme gene in the positive clone obtained by enzyme digestion was connected to the expression vector pET29a that had been treated in the same way, and the connection product was transformed into E.coli DH5α competent cells, cultivated on LB-resistant medium, picked a single colony, and ...
Embodiment 2
[0056] Fermentation culture of recombinant Escherichia coli:
[0057] The recombinant Escherichia coli was inoculated into LB liquid medium: yeast extract powder 5g / L, tryptone 10g / L, NaCl 10g / L), and the cells were activated at 37°C and 200rpm. Then transferred to the seed medium, 37 ° C, 200 rpm overnight shaking culture. Inoculate 1.5L fermentation medium with 1% inoculum amount, culture cells to OD at 37°C with 200rpm aeration 620 After = 0.6-0.8, the inducer IPTG was added to induce overnight, and the bacteria were collected by centrifugation. As the recombinant Escherichia coli used in the following examples of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com