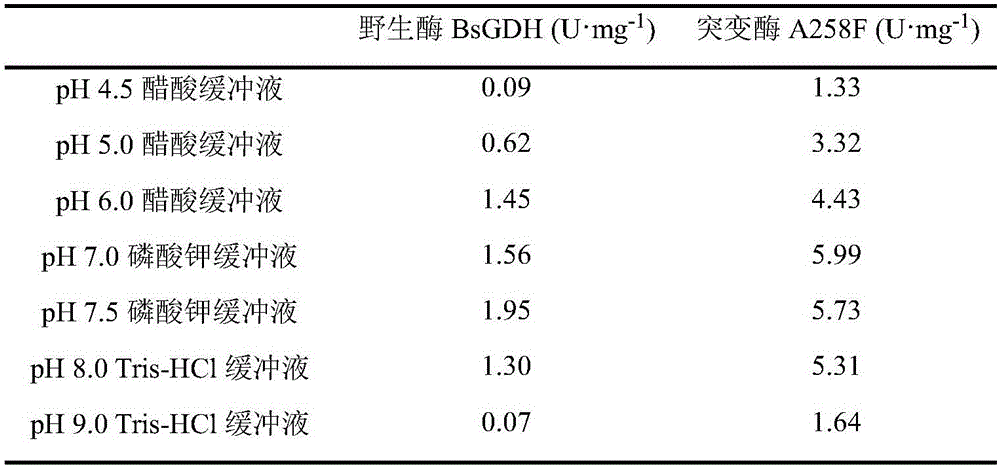
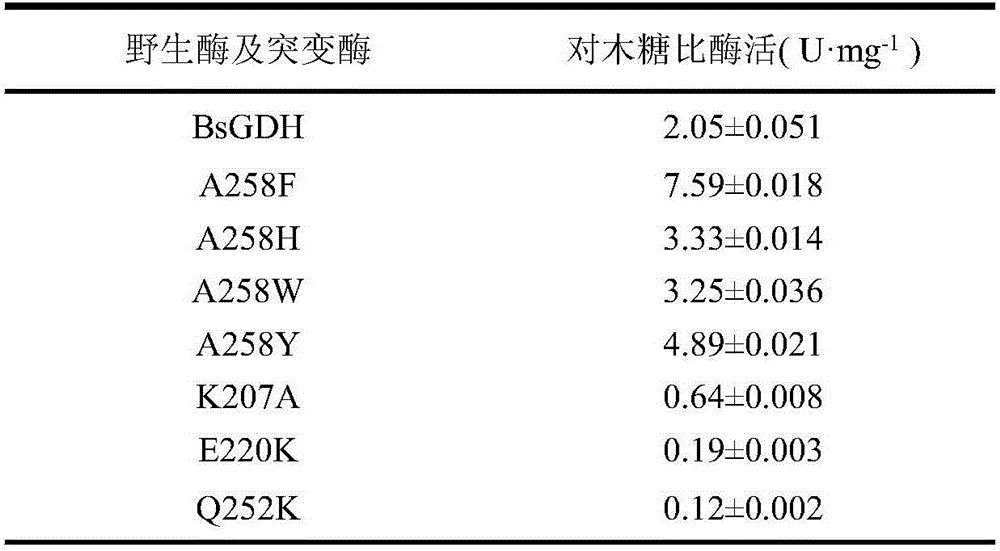
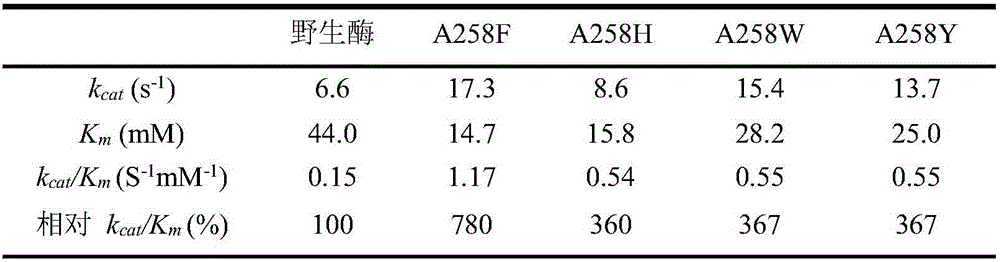
Glucose dehydrogenase mutant with improved specific enzyme activity of catalytic xylose
A technology of glucose dehydrogenase and mutants, applied in the directions of oxidoreductase, enzymes, biochemical equipment and methods, etc., can solve the problems such as the ineffective utilization of xylose resources, and achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Construction of recombinant bacteria expressing mutant A258F
[0028] 1. Acquisition of plasmid pET-GDH
[0029] The medium composition of recombinant E.coli BL21(DE3) / pET-GDH is: peptone 1%, yeast extract 0.5%, NaCl 1%.
[0030] The recombinant bacteria were inoculated in a test tube with a liquid volume of 5 mL of culture medium and cultured at 37° C. with shaking at 200 rpm for 8 h. After the cultivation, the cells were centrifuged at 12,000rpm for 1min and the cells were collected, and the plasmid pET-GDH was extracted using the plasmid extraction kit Mini-Plasmid RapidIsolation Kit (Beijing Biotech Biogene Technology Co., Ltd.); wherein the plasmid pET-GDH The amino acid sequence of glucose dehydrogenase GDH is shown in SEQ ID NO: 1 (the nucleotide sequence is shown in SEQ ID NO: 2).
[0031] 2. Construction of recombinant Escherichia coli E.coli BL21(DE3) / pET-GDH(A258F)
[0032] (1) Acquisition of A258F full-length gene:
[0033] Synthetic primer...
Embodiment 2
[0055] Embodiment 2: Induced expression culture of recombinant bacteria
[0056] LB medium: peptone 1%, yeast extract 0.5%, NaCl 1%, pH 7.0. When needed, add kanamycin sulfate (50 μg·mL) before use -1 ), add 1.5% agar powder to the solid medium.
[0057] Pick a single colony of positive clones and inoculate in 10mL containing 50μg·mL -1 In the LB liquid medium of kanamycin sulfate, culture overnight at 37° C. with shaking at 200 rpm. Take 10mL culture medium and transfer to 1L containing 50μg·mL -1 In the LB liquid medium of kanamycin sulfate, shake culture at 37°C and 200rpm to OD 600 about 1.0. Isopropyl-B-D-thiogalactopyranoside at a final concentration of 0.1 mM was added to the culture, and induction culture was carried out at 37° C. for 12 h.
Embodiment 3
[0058] Embodiment 3: Induced expression culture of recombinant bacteria
[0059] Induced expression: The composition of LB medium is the same as that in Example 2, and a single colony of positive clones is picked and inoculated in 10 mL containing 50 μg·mL -1 In the LB liquid medium of kanamycin sulfate, culture overnight at 37° C. with shaking at 200 rpm. Preserve two branches of glycerol bacteria (each containing 1mL bacterial solution, 15% glycerol), and transfer 1mL culture solution to 50mL containing 50μg·mL -1 In the LB liquid medium of kanamycin sulfate, shake culture at 37°C and 200rpm to OD 600 about 1.0. Isopropyl-B-D-thiogalactopyranoside at a final concentration of 0.1 mM was added to the culture, and induction culture was carried out at 37° C. for 12 h. Bacteria were collected, dissolved in 20mM phosphate buffer (pH 7.0), and ultrasonically disrupted for 20min with a working time of 1s and a resting time of 3s. The crushed bacteria were processed at 12,000 rpm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com