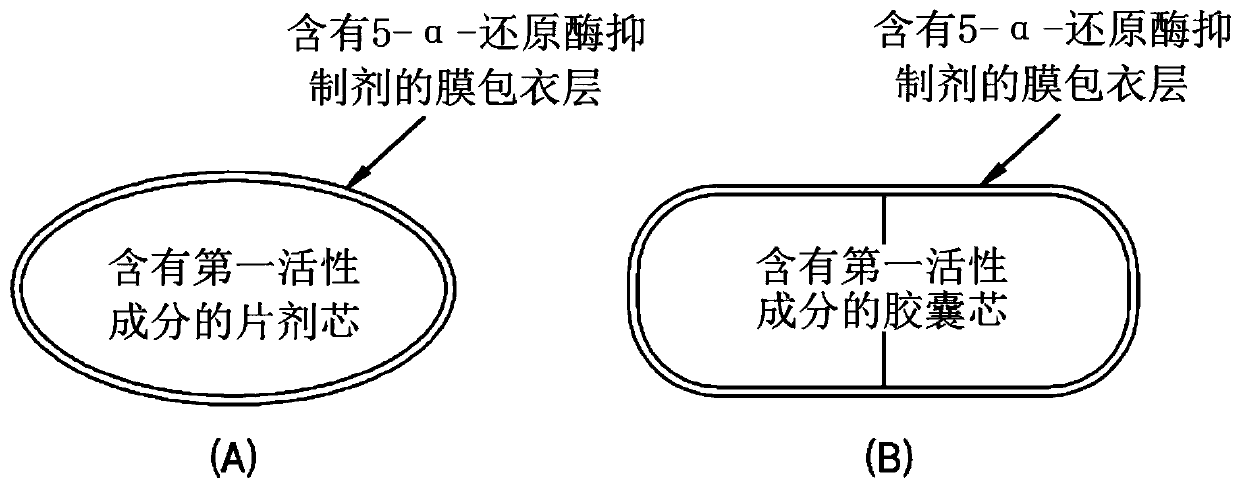
Combination formulation comprising film coating layer containing active ingredient
A technology of active ingredients and compound preparations, which is applied in the field of preparation of compound preparations, can solve problems such as difficulty in ensuring sufficient efficacy and quality of compound preparations, inability to commercialize preparations, separation or rupture of film coating layers, etc., and achieve high salable product quality, The effect of ensuring bioavailability and increasing convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] In the preparation of the core, the core may be any core that can be used as a core in the pharmaceutical field, for example in the form of a tablet, a hard capsule or a soft capsule. However, the embodiments are not limited thereto. In the preparation of the core, any commercially available preparation that can be used as the core can be used, or the core used in the present invention can be directly prepared. The core can be prepared using known techniques in the pharmaceutical field selected by those of ordinary skill in the art depending on the type of core.
[0069] In preparing the coating solution containing the second active ingredient, the solvent can be any pharmaceutically available solvent that can dissolve the second active ingredient, polyvinyl alcohol-polyethylene glycol graft copolymer and polyvinyl alcohol. For example, the solvent can be water, ethanol, methanol, chloroform, dimethyl sulfoxide (DMSO) or any combination thereof. In some embodiments, t...
Embodiment 1-10
[0074] Examples 1-10: Including tamsulosin hydrochloride gel coated with a film coating layer containing a 5-alpha-reductase inhibitor Preparation of compound formulation of capsule (1)
[0075] A coating solution was prepared by mixing coating materials (Examples 1-10) having different compositions shown in Table 1 with an ethanol-water mixed solution (ethanol:water=1:1 (v / v)), and then By using a pan coater (SFC-30, available from Sejong Co., Ltd.) coated on tamsulosin hydrochloride containing Capsules (available from Hanmi Pharmaceutical Co., Ltd., Korea). The prepared capsules were dried at about 35° C. for about 30 minutes, thereby preparing a composite preparation including a tamsulosin capsule core coated with a film coating layer containing a 5-α-reductase inhibitor.
Embodiment 11 and 12
[0084] Examples 11 and 12: Including Tamsulosin OD Tablets Coated with a Film Coating Containing a 5-alpha-Reductase Inhibitor Preparation of core compound formulations
[0085] A coating solution was prepared by mixing coating materials (Examples 11 and 12) having different compositions shown in Table 2 with an ethanol-water mixed solution (ethanol:water=1:1 (v / v)), and then By using a pan coater (SFC-30, available from Sejong Co., Ltd.) in the mixture containing tamsulosin hydrochloride OD tablets (available from Hanmi Pharmaceutical Co., Ltd., Korea) were coated. The prepared composite tablet was dried at about 35° C. for about 30 minutes, thereby preparing a tablet comprising a film coating layer containing a 5-α-reductase inhibitor. Composite formulation of OD tablet cores.
[0086] [Table 2]
[0087]
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com