Method and kit for in vitro detection of tumor neoantigen specificity T cells and tumor vaccine
An in vitro detection and specific technology, applied in the field of molecular immunology and cellular immunology, can solve the problems of high uncertainty, high detection cost, low sensitivity, etc., and achieve the effect of low cost, simple detection method and clear results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1. Prediction and synthesis of tumor neoantigens
[0065] Take the isolated tumor tissue for genome analysis to find out non-synonymous gene mutation sites (including new amino acid sequences formed by nucleic acid point mutations, insertions or deletions), and use the antigen prediction software netMHC4 based on the inclusion of the non-synonymous The binding ability of the antigen at the gene mutation site to the corresponding major histocompatibility molecule is to select the antigen peptide sequence with the best binding ability as the candidate tumor neoantigen peptide. The ability of a candidate tumor neoantigen peptide to bind to the corresponding MHC is indicated by the minimum concentration of the antigen peptide required to achieve stable MHC-antigen peptide binding. The lower the concentration, the more stable the binding of the antigen peptide to the MHC molecule, the stronger its antigenicity, and the better it can stimulate the proliferation of spe...
Embodiment 2
[0075] Example 2 Tumor neoantigen peptide pre-stimulation
[0076] In the same case of tumor patient, 10mL of peripheral venous blood was extracted with a heparin sodium anticoagulant tube under informed conditions. Lymphocyte separation medium (Fresenius Kabi Norge AS, Lymphoprep TM ) After the peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation, they were suspended in fresh serum-free medium (AIM-V from Life Technology Company) with a cell density of 1-3x10 6 / mL. In the serum-free medium, add the above tumor neoantigen peptide or the corresponding native peptide (1-10 μg / mL), at 37°C, 5% CO 2Cultivate in the incubator for 36-48h. The purpose of detecting the immune response of the patient's PBMC to the native peptide is to test whether the immune response to the tumor neoantigen is targeting the mutant sequence, so as to determine that the tumor neoantigen-specific T cells only specifically target and recognize the mutant cells, t...
Embodiment 3
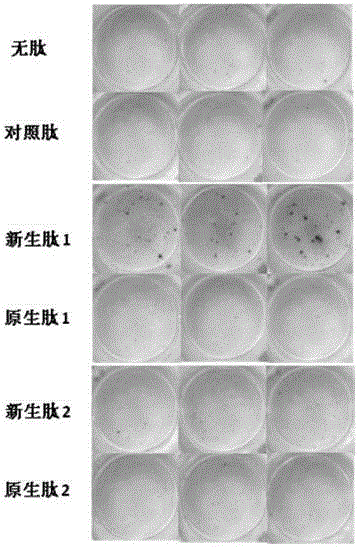
[0077] Example 3 Using IFNγ ELISpot method to detect tumor neoantigen-specific T cells that recognize the tumor neoantigen (U-CyTech BV, CT230-PR5)
[0078] For the operation procedure, refer to the manual of the kit. The specific experimental process is as follows:
[0079] Wet the Elispot plate with 70% ethanol aqueous solution at room temperature for 1 hour, then wash it twice with PBS, add IFNγ-coated antibody to coat the Elispot plate, cover the plate and incubate overnight at 4°C, wash it 3 times with PBS, and add blocking solution to block Cover the unbound position of the IFNγ antibody and incubate at 37°C for 1 hour;
[0080] Adjust the pre-stimulated PBMC concentration to 1-3x10 6 / ml, add corresponding 1-10ug / mL tumor neoantigen (neoplastic peptide 1, neonatal peptide 2 in Table 1), tumor native antigen (native peptide 1 and native peptide 2 in Table 1), control peptide ( For example, a peptide of the envelope protein of HIV virus) serum-free cell culture medium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com