Triple PCR (Polymerase Chain Reaction) detection method for simultaneously detecting plurality of types of pathogens
A detection method and sequence technology, applied in the field of PCR detection, can solve the problems of inappropriate epidemiological investigation, time-consuming, cumbersome process, etc., and achieve the effects of rapid detection standardization, shortening detection time, and improving detection efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
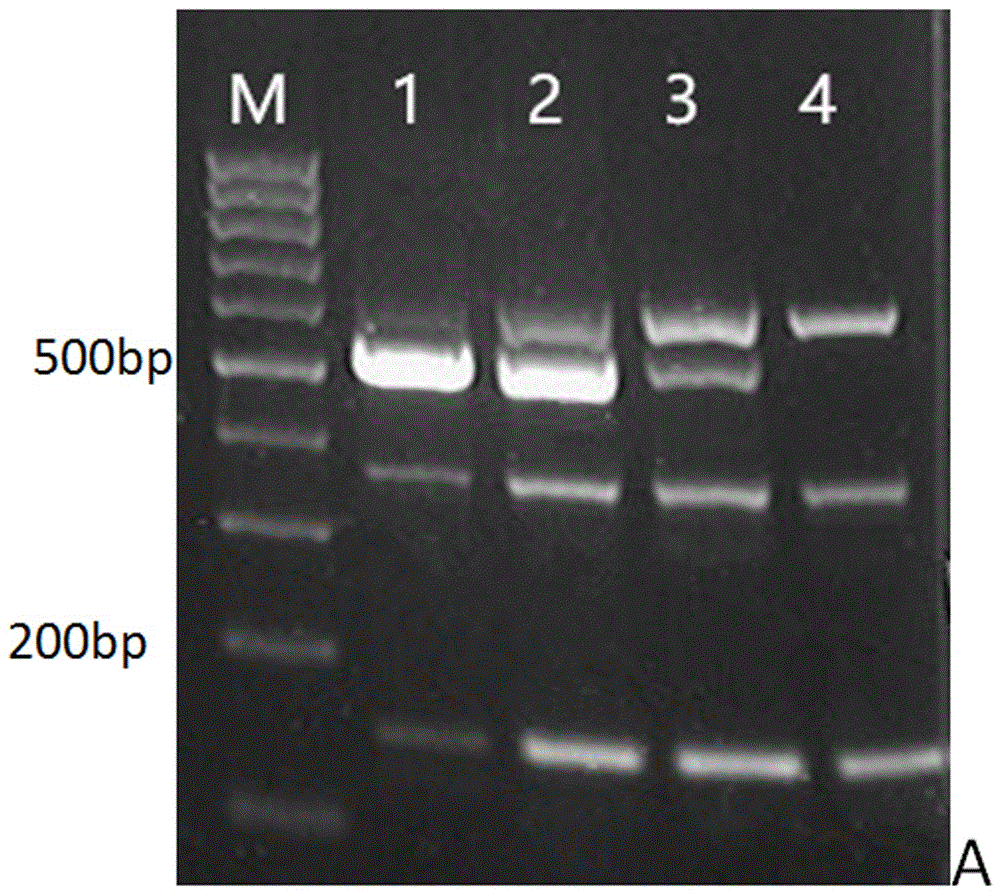
[0027] Example 1 Optimization of the annealing temperature of the triple PCR system of Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiella pneumoniae
[0028] (1) Sample pretreatment
[0029] The standard Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiella pneumoniae were cultured in the corresponding medium or culture solution, among which Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiella pneumoniae were cultured for 24h, and the bacteria were collected. The corresponding DNA samples were extracted from the solution or colony.
[0030] (2) Bacterial DNA extraction
[0031] The present invention adopts the bacterial genome DNA extraction kit to extract DNA, and the steps are as follows:
[0032] Dissolve the above-mentioned 3 kinds of pathogen bacteria liquids or colonies in buffer GA, shake and suspend;
[0033] Add 20 μl Proteinase K solution, mix well, add 220 μl Buffer GB, shake for 15 s, 70°C for 10 min, add 220 μl absolute ethanol, sha...
Embodiment 2
[0044] Example 2 Detection of triple PCR artificial samples of Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiella pneumoniae
[0045] (1) Sample pretreatment
[0046] Take a sample feces from the animal to be tested.
[0047] (2) Bacterial DNA extraction
[0048] Same as Example 1
[0049] (3) Establishment of multiplex PCR reaction system
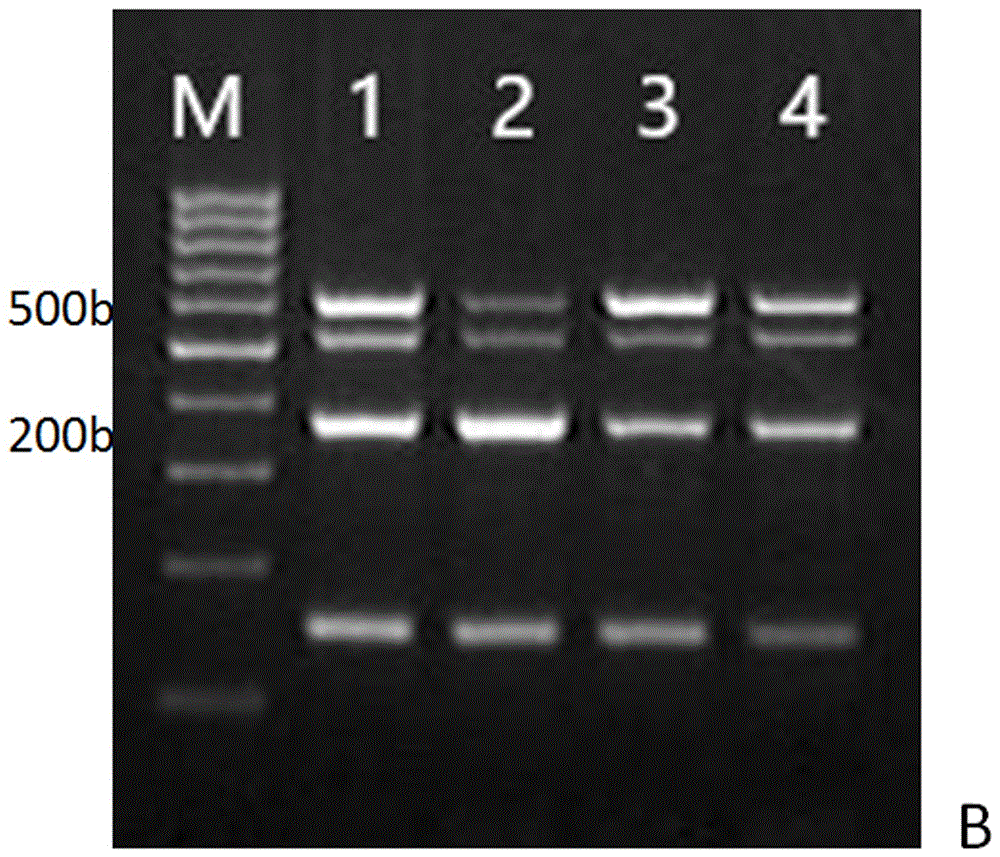
[0050] The PCR reaction system is 50 μl, including: Mix 25 μl, primers 6.5 μl, of which, the universal upstream and downstream primers for Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae and bacteria are 1 μl, 1 μl, 1 μl, 0.25 μl, respectively, DNA The positive control was 3 μl, the negative control was 0 μl, the sample feces were 3 μl each, and the rest of the sterile water was supplemented to 50 μl. Reaction conditions: pre-denaturation at 94°C for 5 min; 30 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s; final extension at 72°C for 7 min. 10 μl of PCR products were electrophoresed in 2.5% a...
Embodiment 3
[0053] Example 3 Detection of triple PCR samples of Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiella pneumoniae
[0054] (1) Sample pretreatment
[0055] Take sample feces from the live animal to be tested.
[0056] (2) Bacterial DNA extraction
[0057] Same as Example 1
[0058] (3) Establishment of multiplex PCR reaction system
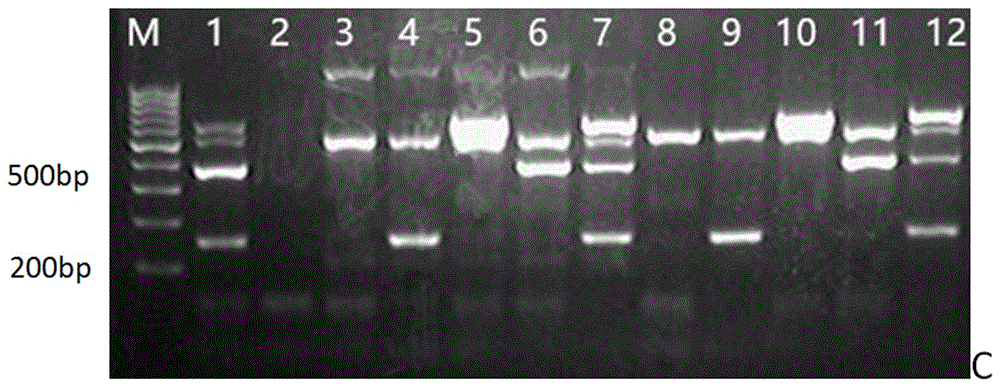
[0059] The PCR reaction system is 50 μl, including: Mix 25 μl, primers 6.5 μl (1 μl, 1 μl, 1 μl, 0.25 μl of universal upstream and downstream primers for Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, and bacteria, respectively), DNAs are The positive control was 3 μl, the negative control was 0 μl, the sample feces were 3 μl each, and the rest of the sterile water was supplemented to 50 μl. Reaction conditions: pre-denaturation at 94°C for 5 min; 30 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s; final extension at 72°C for 7 min. 10 μl of PCR products were electrophoresed in 2.5% agarose gel at 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com