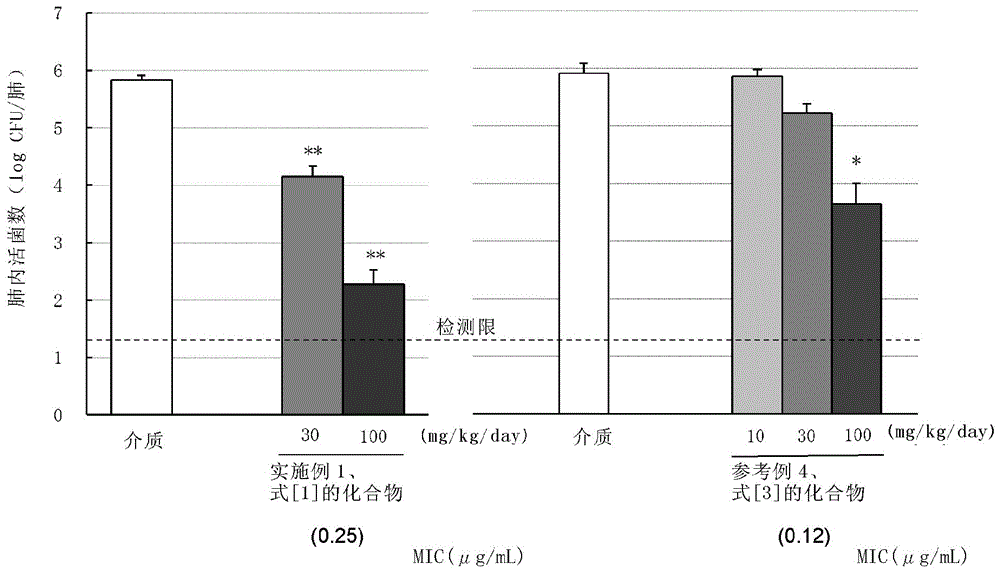
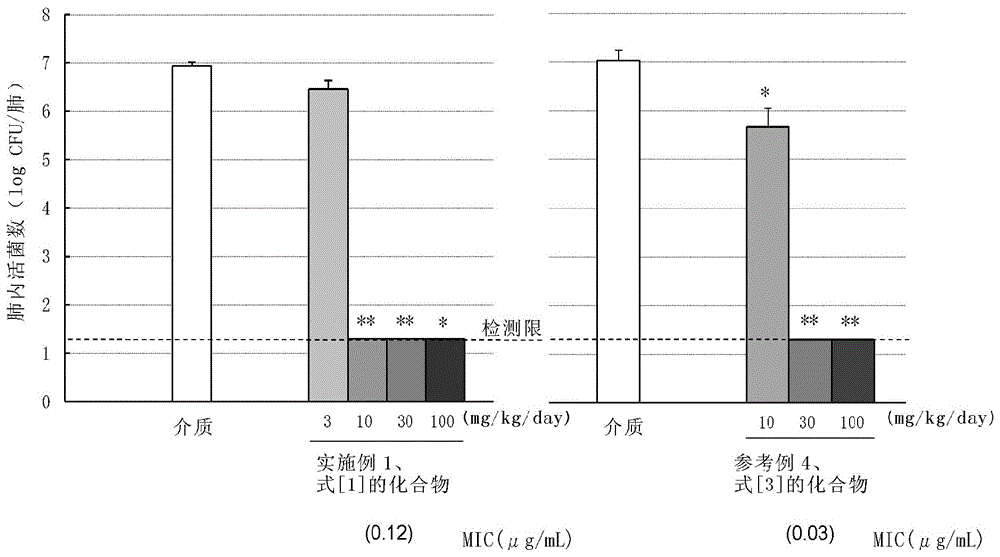
C-4''-substituted macrolide compound
A technology of compounds and hydrates, applied in the direction of sugar derivatives, organic chemistry, antibacterial drugs, etc., can solve the problems of unstable dynamics in vivo and achieve good antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0040]Hereinafter, the present invention will be further described in detail through reference examples, examples, and test examples. The method for synthesizing the compound of the present invention is not limited to the following methods, and methods known to those skilled in the art, such as switching the order of the steps and protecting and deprotecting functional groups, can also be used.
[0041] Each equipment data of following reference example, embodiment record is measured by following measurement equipment;
[0042] NMR spectrum: JEOL Ltd. JNM-ECA600 (600MHz), JEOL Ltd. JNM-ECA500 (500MHz)
[0043] MS spectrum: Shimadzu Corporation LCMS-2010EV or Micromass Company Platform LC.
[0044] In the following reference examples and examples, high-performance liquid chromatography-mass spectrometry (LCMS) is determined by the following conditions;
[0045] Determination equipment: Agilent 2900 and Agilent 6150
[0046] Column: Waters (Waters) Acquity CSH C18, 1.7μm, φ2....
reference example 1
[0066] Reference Example 1 Synthesis of N,N-diisopropyl-N-methylethane-1,2-diamine
[0067]
[0068]
[0069] To 135 mL of a methanol solution of 8.9 mol / L methylamine, 72 mL of a methanol solution of 24.0 g of diisopropylaminochloroethane hydrochloride was added dropwise under ice-cooling, and stirred at room temperature for 20 minutes. The reaction liquid was concentrated under reduced pressure, the obtained residue was dissolved in chloroform, and a 2 mol / L sodium hydroxide aqueous solution was added under ice-cooling. The reaction solution was extracted twice with chloroform, the organic layer was concentrated under reduced pressure, and the resulting residue was purified by amino silica gel column chromatography (hexane:chloroform=5:1 to chloroform only) to obtain 19.4 g of the title compound;
[0070] MS(ESI) m / z= 159 [M+H]+
[0071] 1H-NMR (400 MHz, CDCl 3 ) δ(ppm) : 0.99 (d, J=1.71 Hz, 6 H) 1.00 (d, J=1.71Hz, 6 H) 2.43 (s, 3 H) 2.54 -2.57 (m, 4 H) 2.96 -3.03 ( ...
reference example 2
[0072] Reference example 2 Synthesis of 2-amino-N-ethylacetamide
[0073]
[0074]
[0075] (1) Add 108mL of 70% ethylamine aqueous solution to 1.0L of N-(benzyloxycarbonyl)glycine 209g in chloroform, and add 1-ethyl-3-(3-dimethylaminopropyl) carbon under ice-cooling conditions After adding 249 g of diimine hydrochloride, it was stirred overnight at room temperature. Saturated aqueous sodium bicarbonate solution was added to the reaction liquid, followed by extraction with chloroform. The organic layer was concentrated under reduced pressure, the obtained residue was suspended in 400 mL of ethyl acetate, 200 mL of hexane was added and stirred, and the generated solid was collected by filtration to obtain 150 g of an amide compound.
[0076]
[0077]
[0078] (2) 15 g of 10% palladium carbon was added to 630 mL of methanol solution of 150 g of the amide compound obtained in Reference Example 2-(1) above, and stirred at room temperature for 6 days under a hydrogen atm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com