Preparation method of a carbon-coated transition metal phosphide composite and its application in oxygen evolution reaction
A technology of transition metals and composite materials, applied in chemical instruments and methods, physical/chemical process catalysts, electrolytic processes, etc., can solve problems such as flammability and toxicity, affect electrocatalytic activity, etc., achieve low price, improve catalytic activity, and prepare The effect of simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
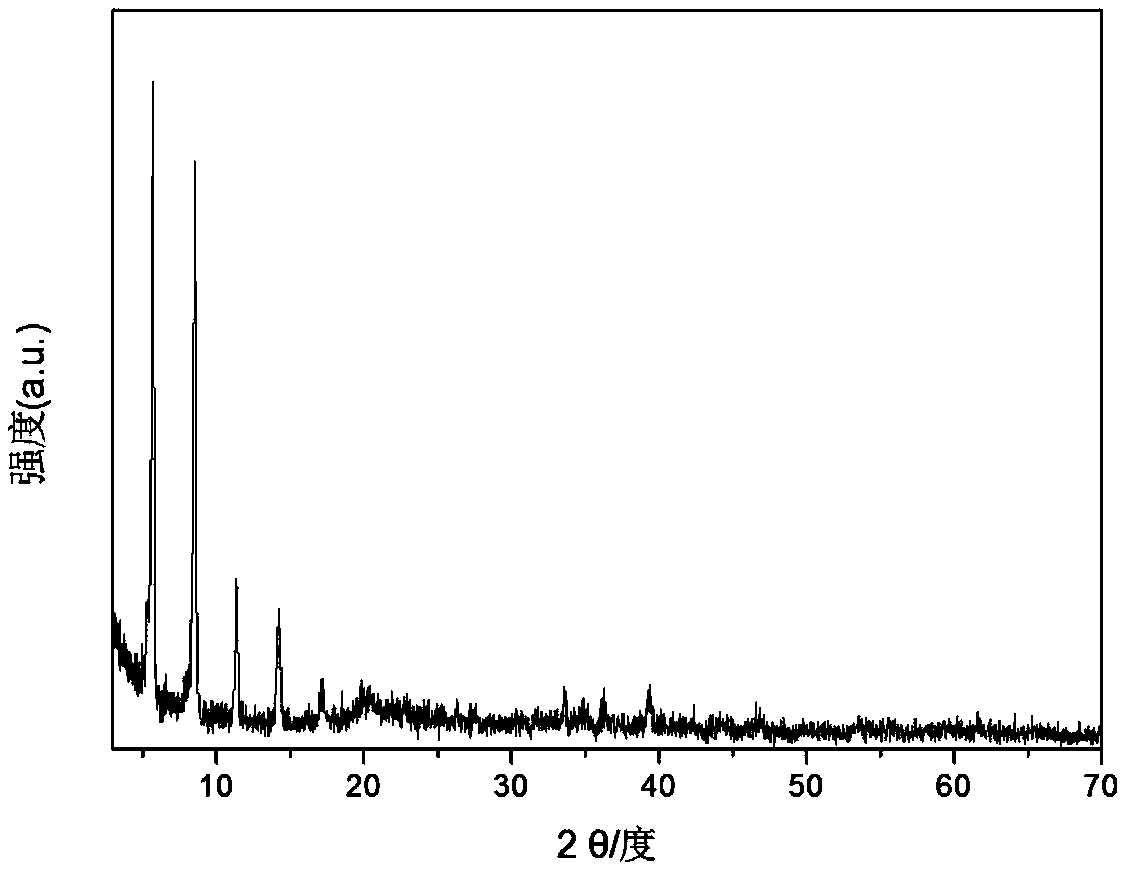
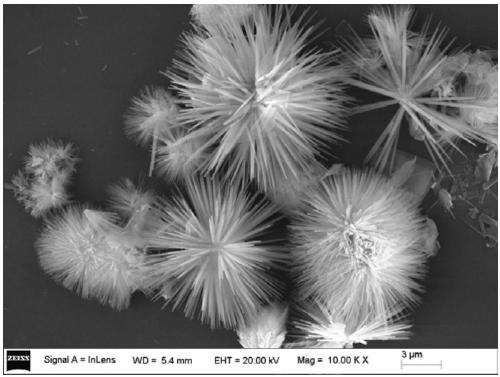
[0026] A. Add 7.2mmol Co(NO 3 ) 2 ·6H 2 O. Add 24mmol urea into 200mL beaker a, add 150mL carbon dioxide-free water, and dissolve it by ultrasonic. Add 3.6mmol monododecyl phosphate (SDP) into 150mL beaker b, add 90mL carbon dioxide-free water, and ultrasonically dissolve. Add the solution in bottle b, which is evenly mixed and dispersed, into bottle a, protect it with an inert atmosphere, stir for a period of time, then transfer the solution in a to an autoclave, crystallize at 150°C for 6 hours, and use it repeatedly to remove carbon dioxide after crystallization Washing with water and ethanol and centrifugation to pH 7, followed by drying at 70 °C for 24 h yielded highly dispersed needle-like SDP-intercalated Co(OH) 2 Precursor (XRD, SEM picture see figure 1 and figure 2 );
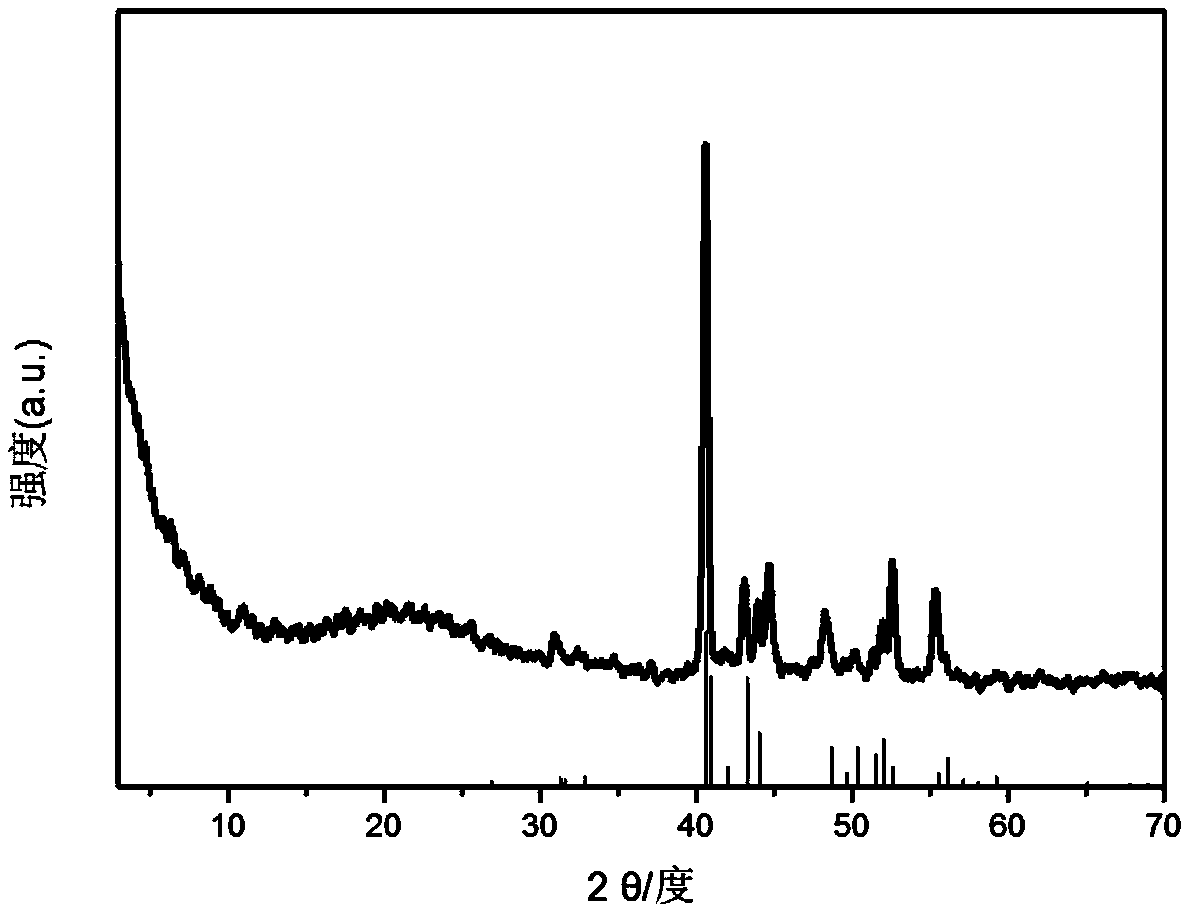
[0027] B. Co(OH) intercalated with the highly dispersed acicular SDP prepared in step A 2 The precursor is placed in a high-temperature atmosphere furnace, and H 2 / Ar gas, the flow rate is 60...
Embodiment 2
[0034] Other conditions are the same as in Example 1, except that the inorganic metal salt is nickel nitrate hexahydrate. The electrocatalytic performance test results are summarized in Table 1.
Embodiment 3
[0036] Other conditions are the same as in Example 1, except that the inorganic metal salt is cobalt sulfate hexahydrate. The electrocatalytic performance test results are summarized in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com