Fusion protein Slit2D2 (C386S)-HAS and application thereof in treating fibrosis diseases
一种融合蛋白、蛋白的技术,应用在生物医药领域,能够解决半衰期短等问题,达到分子量小、提高稳定性、组织渗透性好的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
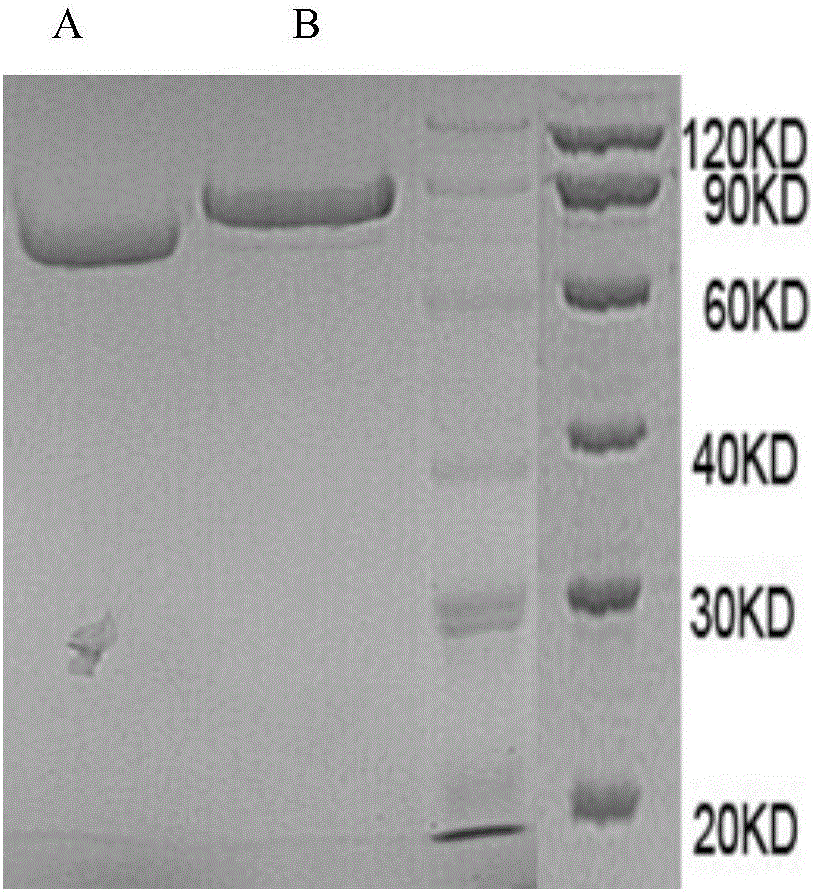
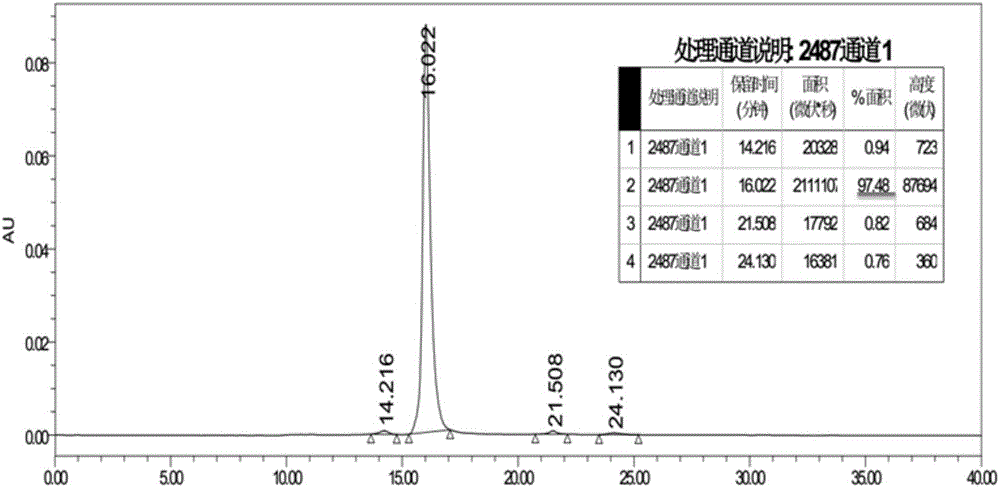
[0072] Example 1 Preparation of fusion protein Slit2D2(C386S)-HSA
[0073] According to the known Slit2 sequence [GenBank:EAW92793.1], the second structural domain Slit2D2 of Slit2 was designed and constructed, and Slit2D2 (C386S) was designed as shown in SEQ ID NO: 1, and then Slit2D2 (C386S) and Slit2D2 (C386S) were designed - The coding sequences of HSA are respectively shown in SEQ ID NO: 2 and SEQ ID NO: 4.
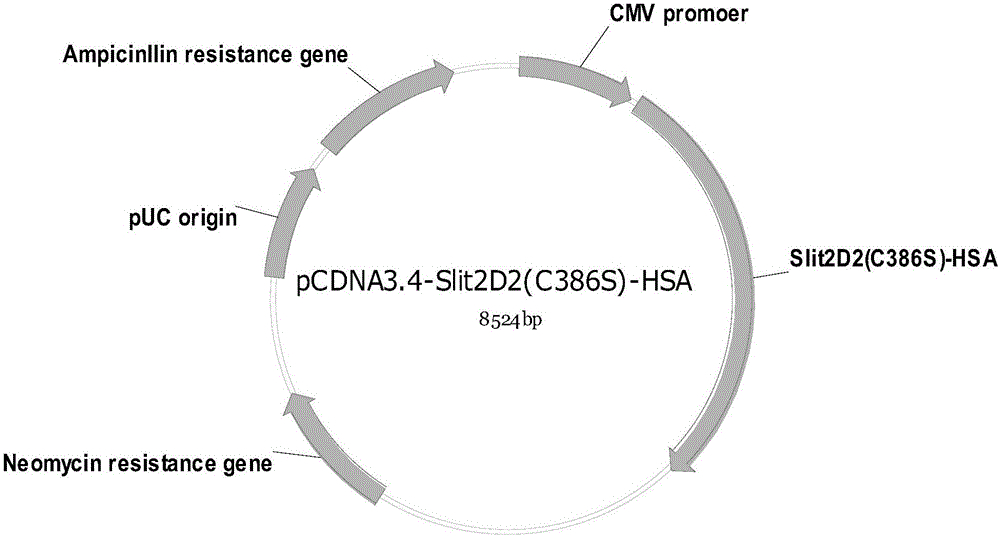
[0074] The coding sequence of Slit2D2(C386S)-HSA was obtained by whole gene synthesis, and inserted into the expression vector pCDNA3.4 (brand: Thermo, catalog number: A14697) by T / A cloning, and the recombinant vector pCDNA3.4-Slit2D2(C386S)- HSA profile such as figure 1 shown. The above-mentioned recombinant expression vector was transformed into Escherichia coli TOP10, and then transferred to a solid medium containing ampicillin (AMP) for propagation, positive clones were screened, and the success of vector construction and species preservation were confirmed by...
Embodiment 2
[0077] Example 2 Determination of the affinity of the fusion protein to the target protein Robo1 by SPR
[0078] Utilize the SPR (Surface Plasmon resonance BIAcore200) method to detect the affinity constant between the protein and the Robo1 protein, bind the Robo1 (ORIGEN company, product number: TP327713) protein on the CM5 chip, and analyze the fusion protein Slit2D2(C386S)-HSA (Example 1), the interaction between Slit2D2-HSA (see patent application PCT / CN2015 / 092079) and the receptor protein Robo1. Kinetic measurements were performed according to the protocol of Canziani et al. (2004, Anal. Biochem. 325:301-307). At the same time, the affinity between Slit2N protein (Slit2N is a protein with a molecular weight of about 120 KDa at the N-terminal of slit2 protein) and Robo1 protein was also determined by the same method. The results are shown in Table 1.
[0079] Table 1 The results of SPR determination of the affinity of the fusion protein to the receptor Robo1
[0080] ...
Embodiment 3E
[0082] Embodiment 3ELISA measures protein stability
[0083] 1. Reagents:
[0084] Neutroavidin-HRP dilution;
[0085] Coating buffer: -0.16%Na 2 CO 3 ;-0.3% NaHCO 3 ;-pH9.8;
[0086] Washing buffer: PBS containing -0.1% Tween20;
[0087] Blocking buffer: washing buffer containing -1% Goat Serum;
[0088] TMB: purchased from Shanghai Biyuntian Biotechnology Co., Ltd.;
[0089] Stop solution (Stop solution): purchased from Shanghai Biyuntian Biotechnology Co., Ltd.;
[0090] NOTE: All antibodies are diluted in blocking buffer.
[0091] 2. Experimental process:
[0092] Robo1 protein was diluted to 1 μg / ml, coated with 100 μl / well, and left overnight at 4°C. Wash the plate 3 times with Washing buffer. Blocking buffer (200μl / well) was blocked at room temperature for 2 hours. Wash the plate 3 times with Washing buffer. The samples to be tested (fusion protein Slit2D2(C386S)-HSA (prepared in Example 1), Slit2D2-HSA (see patent application PCT / CN2015 / 092079) 100 μl) wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com