Imidazolecarboxylic acid complex and synthesis method and application thereof
A technology of imidazole carboxylic acid and synthesis method, which is applied in the field of coordination chemistry and achieves the effects of mild reaction conditions, simple synthesis method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
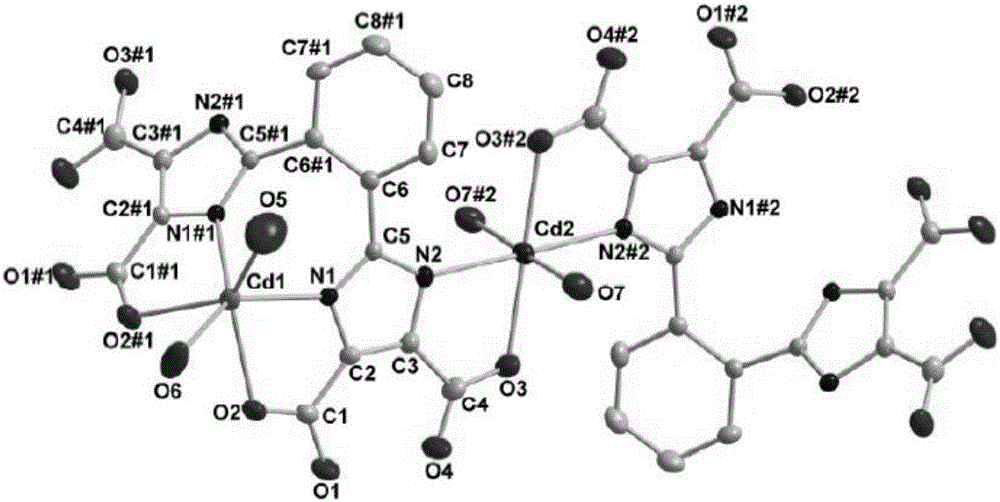
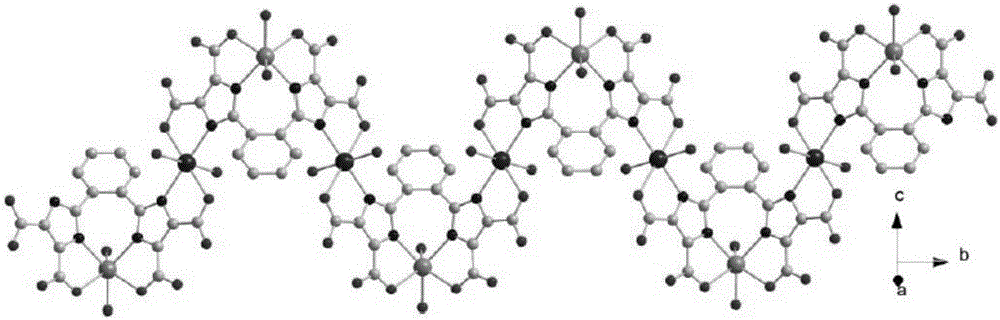
[0040] Example 1 Synthesis of 2,2'-(1,2-phenyl)bis(1H-imidazole-4,5-dicarboxylic acid)
[0041] 1. Synthesis of 1,2-bis(2-benzimidazolyl)benzene
[0042]Mix 4.758g (0.044mol) of o-phenylenediamine and 3.3226g (0.02mol) of phthalic acid, then add 40ml of 85% phosphoric acid (mass fraction), intermittently heat up to 180°C, stir and reflux for 6h, cool to room temperature, and then Pour into 160ml of ice water, leave at room temperature for 6h, and filter with suction to obtain a blue-green solid. Recrystallize the blue-green solid with a mixture of methanol and dimethylformamide (DMF) (volume ratio 1:1.5) to obtain white needle-like crystals, filter, wash, and dry in vacuo to obtain 2.16 g of 1,2- Bis(2-benzoimidazolyl)benzene (OBMB), yield: 76.59%.
[0043] The intermittent temperature rise is as follows: from room temperature to 140°C, every time the temperature rises by 10°C, keep warm for 5 minutes (when the temperature rises to 140°C for the last time, if the temperature...
Embodiment 2
[0046] Example 2 Structural characterization of 2,2'-(1,2-phenyl)bis(1H-imidazole-4,5-dicarboxylic acid)
[0047] 1. NMR spectrum
[0048] Adopt NMR spectrometer Avance III (500MHz), TMS is internal standard, measure the 2,2'-(1,2-phenyl) bis(1H-imidazole-4,5-dicarboxylic acid) that embodiment 1 obtains Hydrogen spectrum, the analysis result is: 1 H NMR (500 MHz, DMSO) δ 7.97 (dd, J=5.8, 3.3 Hz, 2H), 7.78 (dd, J=5.8, 3.3 Hz, 2H).
[0049] 2. Elemental analysis
[0050] The elemental analysis of 2,2'-(1,2-phenyl)bis(1H-imidazole-4,5-dicarboxylic acid) obtained in Example 1 was carried out with an American Thermo FLASH EA 1112 elemental analyzer, and the analysis result was : The measured value (%) of C, H, N is respectively: C49.65, H 2.71, N 14.62; According to molecular formula C 16 h 10 o 8 N 4 (M r =386.27) The theoretical values (%) of C, H, and N obtained by calculation are respectively: C 49.75, H 2.61, N 14.50. It can be known from the elemental analysis resu...
Embodiment 3
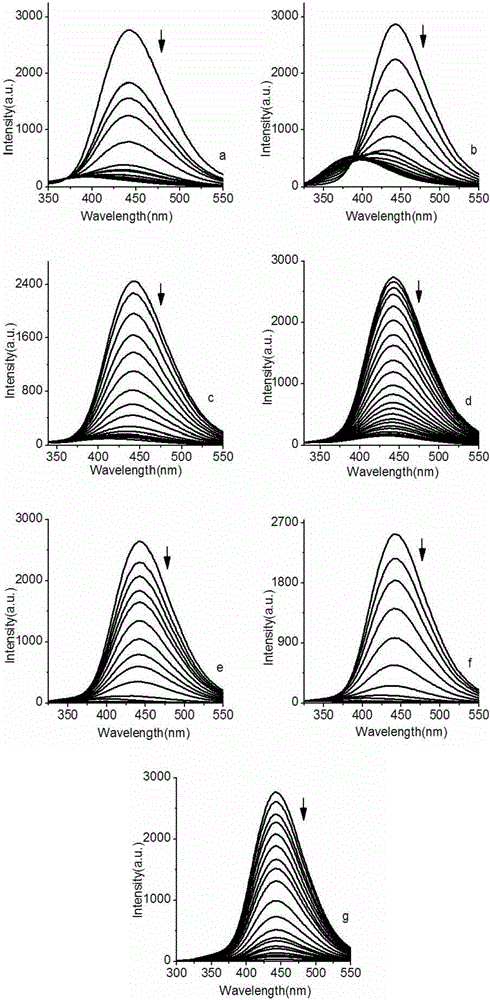
[0051] Example 3 Sensing performance detection of 2,2'-(1,2-phenyl)bis(1H-imidazole-4,5-dicarboxylic acid)
[0052] Test instrument: Fluorescence spectrophotometer, model F7000, produced by Hitachi, Japan.
[0053] Preparation of reagents:
[0054] 1. Sample solution
[0055] 2,2'-(1,2-phenyl)bis(1H-imidazole-4,5-dicarboxylic acid) solution: weigh 0.0386g 2,2'-(1,2-phenyl)bis(1H- imidazole-4,5-dicarboxylic acid) in a beaker, add 20ml dimethyl sulfoxide (DMSO), stir to dissolve, then transfer to a 100ml volumetric flask, dilute to volume with DMSO, and prepare a concentration of 1×10 -3 mol / l 2,2'-(1,2-phenyl)bis(1H-imidazole-4,5-dicarboxylic acid) solution.
[0056] 2. Metal ion storage solution
[0057] Zn 2+ The preparation method of stock solution: weigh Zn(NO 3 ) 2 ·6H 2 O 0.1487g, dissolved in DMSO, formulated as Zn 2+ The concentration is 5×10 -2 mol / l solution.
[0058] Cd 2+ The preparation method of stock solution: weigh Cd(NO 3 ) 2 4H 2 O 0.1542g, diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com