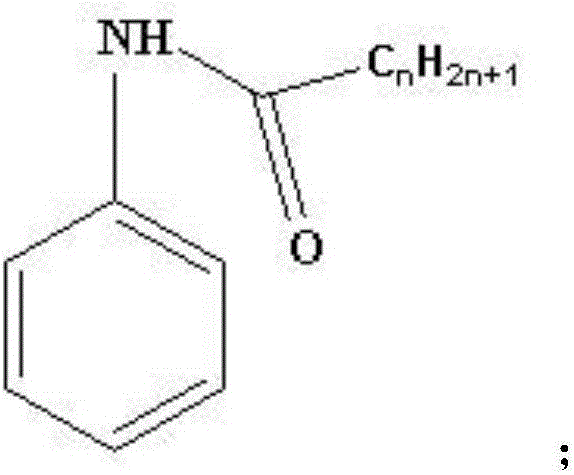
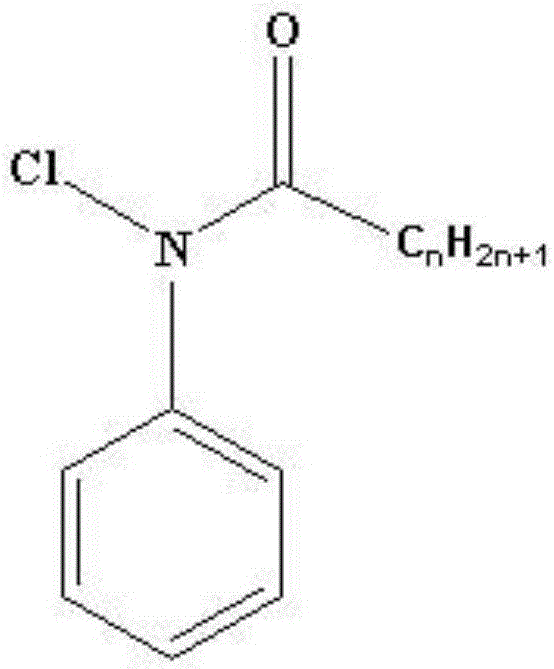
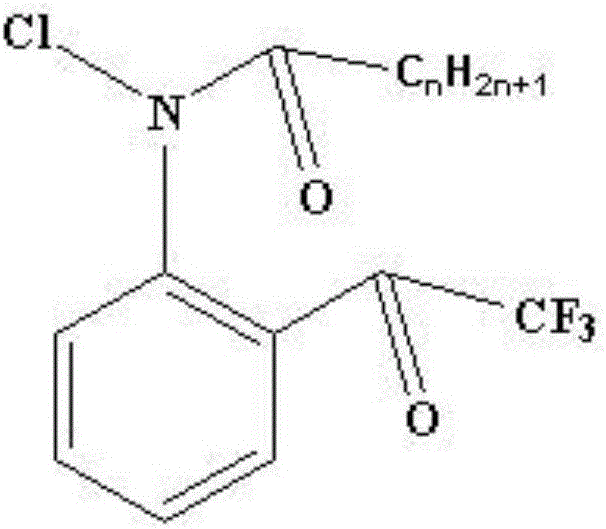
Method for synthesizing 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate
A technology for the synthesis of trifluoroacetylaniline and its synthesis method, which is applied in the field of synthesis of efavirenz intermediates, can solve the problems of high price, achieve the effects of less pollution, lower energy consumption, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of pivalanilide
[0029]
[0030] Add 18.6g of aniline and 150mL of toluene into the reactor, cool down to 0-5°C, add 30g of 30% sodium hydroxide solution, control the temperature at 5-15°C, then add 27.8g of pivaloyl chloride dropwise, and the dropwise addition is completed in about 15 minutes , stirred at 5-15°C for 60 minutes, sampled and tested, separated after the reaction, washed the organic layer twice with water, cooled the organic phase to 0-5°C, kept it warm for 120 minutes, filtered with suction, washed the filter cake with an appropriate amount of water, and drained. After vacuum drying, 34.4 g of pivalanilide was obtained, with a yield of 97.2% and a content of 99.1%.
[0031] Synthesis of N-chloropivalanilide
[0032]
[0033] Put 35.4g of pivalanilide, 50.4g of sodium bicarbonate, 1000mL of diethyl ether and 400mL of water into the reactor, stir well so that the organic phase and the water phase are both clear liquids, then cool down to 0...
Embodiment 2
[0041] Synthesis of Acetanilide
[0042]
[0043] Add 18.6g of aniline and 150mL of toluene into the reactor, cool down to 0-5°C, then add 30g of 30% sodium hydroxide solution, control the temperature at 5-15°C, then add 17g of acetyl chloride dropwise, and the dropwise addition is completed in about 15 minutes. Stir at 5-15°C for 60 minutes, take samples for testing, separate the layers after the reaction, wash the organic layer twice with water, cool the organic phase to 0-5°C, keep it warm for 120 minutes, filter with suction, wash the filter cake with an appropriate amount of water, drain and vacuum After drying, 26.2 g of acetanilide was obtained, with a yield of 96.8% and a content of 99.2%.
[0044] Synthesis of N-Chloroacetanilide
[0045]
[0046] Put 27g of the prepared acetanilide, 50.4g of sodium bicarbonate, 1000mL of ether and 400mL of water into the reactor, stir well to make the organic phase and the water phase both clear, then cool down to 0°C, and slo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com