Orally administered agent for ruminants and ruminant feed containing same
A ruminant, oral technology, applied in the direction of animal feed, molding or processing of animal feed, medical preparations containing active ingredients, etc., can solve the problems of failure to check the breaking strength, difficulty in applying other substances, etc., and achieve reliable High processability and versatility, easy preparation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
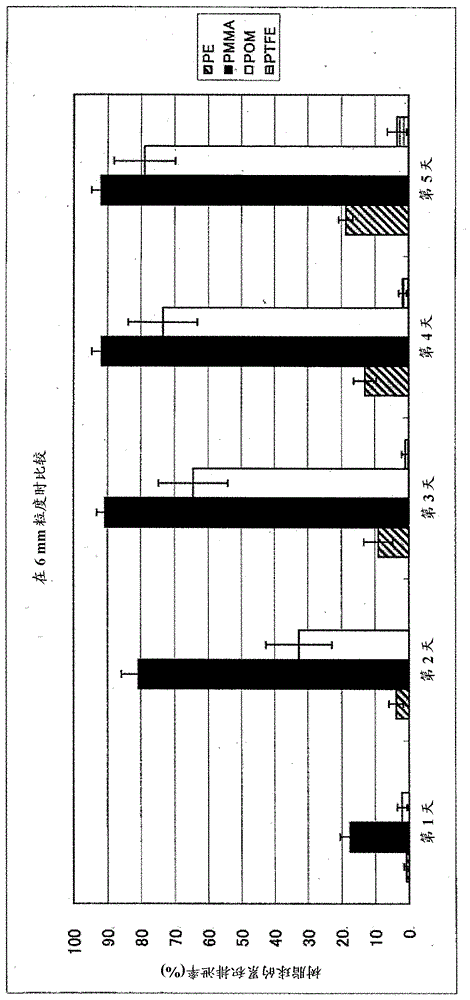
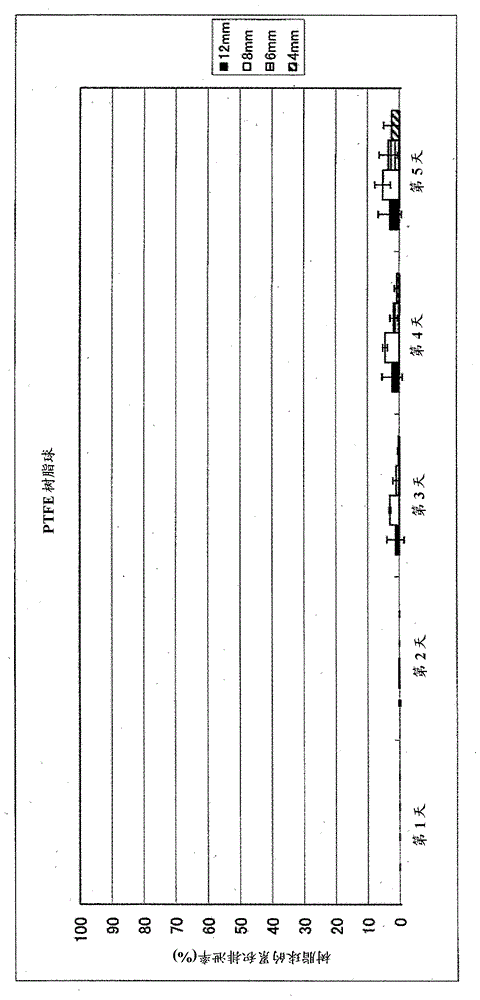
[0103]High-density polyethylene (PE) (specific gravity 0.95), polymethyl methacrylate (PMMA) (specific gravity 1.19), polyoxymethylene (POM) (specific gravity 1.41) in each particle size of 4 mm, 6 mm, 8 mm and 12 mm and polytetrafluoroethylene (PTFE) (specific gravity 2.20) each one hundred commercially available beads were orally administered to 4 lactating cows. The cumulative rate of the number of beads in the intact state (which is excreted in feces) is described in Figure 1-Figure 5 . Figure 1-Figure 4 The cumulative excretion rates for beads of different types of resins, ie, for beads with different specific gravity at each diameter are shown. in addition, figure 1 Data for HDPE beads, figure 2 is the data for polymethylmethacrylate (PMMA) beads, image 3 data for polyoxymethylene (POM) beads, and Figure 4 Data for polytetrafluoroethylene (PTFE) beads. Figure 5 It is a comparison of 4 types of resins in beads with a particle size of 6 mm, ie comparative data...
Embodiment 2
[0107] Production of seamless capsules
[0108] 20 parts of thiamine hydrochloride (manufactured by Tokyo Kasei Kogyo Co., Ltd.) and 20 parts of titanium dioxide were dispersed in 60 parts of Melano H1000S (hydrogenated palm kernel oil produced by Fuji Oil Co., Ltd., which has a melting point of 34°C ( 32-36° C.)) to obtain a core formulation composition (dispersion for preparing cores).
[0109] 30 parts of titanium dioxide were dispersed in a warm solution of 70 parts of extremely hardened rice bran oil (melting point 53°C (52-54°C), Boso oiland fat Co., Ltd.) to obtain an inner shell membrane preparation composition comprising The oily substance of the inner shell membrane.
[0110] As the shell membrane composition for forming the shell membrane, 15 parts of carrageenan (manufactured by Sansho Co., Ltd.), 50.9 parts of dextrin (manufactured by Nippon Starch Chemical Co., Ltd.; DE value less than 10 ), 3 parts of sorbitol (manufactured by Mitsubishi Shoji Foodtech Co., Lt...
Embodiment 3-8 and comparative Embodiment 1-6
[0117] In Examples 3-8 and Comparative Examples 1-6, dry seamless capsules were obtained by the procedures described in Example 2, except that the composition ratios of the core, middle layer and shell film are as shown in Table 1 changes shown.
[0118] Table 1-1
[0119]
[0120] Table 1-2
[0121]
[0122] L-Ascorbic acid: Wako Pure Chemical Industries, Ltd.
[0123] Melano H1000: hydrogenated palm kernel oil obtained from Fuji Oil Co., Ltd., melting point 38°C (36-40°C)
[0124] Melano H3000: hydrogenated palm kernel oil obtained from Fuji Oil Co., Ltd., melting point 42°C (40-44°C)
[0125] Permel 45: middle melting fraction of palm distillate from Fuji oil Co., Ltd., melting point 45°C (43-47°C).
[0126] For each of the obtained dry seamless capsules, use isooctane as a specific gravity standard solution, based on the Archimedes method, measure the specific gravity of the seamless capsules by the following method:
[0127] Put 5 mL of isooctane in a 10 mL mea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Breaking strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com