Dual real-time fluorescence RT-PCR (reverse transcription-polymerase chain reaction) kit for detecting and identifying wild strain and vaccine strain of CSFV (classical swine fever virus) in swine umbilical cord blood and application of dual real-time fluorescence RT-PCR kit
A technology of RT-PCR and swine fever virus, applied in the field of dual real-time fluorescent PCR kits, can solve the problems of inability to effectively distinguish wild strain infection and vaccination, less kits, and long operation time, and achieve direct and effective diagnosis , reliable technical support, and highly specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071]Example 1 The composition of the dual real-time fluorescent RT-PCR kit for detection and identification of swine fever virus wild strain and vaccine strain in pig umbilical cord blood
[0072] (1) 2× fluorescent RT-PCR reaction solution (containing enzyme): the raw material was purchased from Nanjing VAZYME Company; the reaction solution contains UNG enzyme system, which can effectively solve the phenomenon of amplification pollution during the amplification process Pollution ability, etc.;
[0073] (2) RT-PCR primer sets CSFV-F and CSFV-R: synthesized by Shanghai Sangon Bioengineering Co., Ltd., prepared with DEPC water to a concentration of 10 μM.
[0074] Upstream amplification primer CSFV-F: 5'-GCCATGCCCATAGTAGGA-3', which is the sequence of SEQ ID NO:1;
[0075] Downstream amplification primer CSFV-R: 5'-CTACTGACGACTGYCCTGTA-3', which is the sequence of SEQ ID NO:2, wherein Y=C / T;
[0076] (3) Specific fluorescent probes CSFV-LV and CSFV-Wt: synthesized by BGI Cor...
Embodiment 2
[0089] Example 2 The method of using the dual real-time fluorescent RT-PCR kit for detecting and distinguishing the wild strain of classical swine fever virus and the vaccine strain in pig umbilical cord blood
[0090] The method for using the kit of the present invention specifically includes the following steps: (1) sample collection; (2) sample processing; (3) RNA extraction; (4) double real-time fluorescent RT-PCR: utilizing the specific primers and probes designed by the present invention Perform double real-time fluorescent RT-PCR detection; (5) judge the result.
[0091] (1) Sample collection
[0092] 1. Take a clean penicillin bottle and cork, wash it, boil and sterilize it for 30 minutes, dry it and collect it for later use;
[0093] 2. When the piglets are born, squeeze the "cord blood" of all the piglets delivered by each sow into a clean penicillin bottle, 3-5 drops per piglet, and squeeze the "cord blood" into the penicillin bottle bottle, sealed;
[0094] Prec...
Embodiment 3
[0119] Example 3 Verification of the dual real-time fluorescent RT-PCR kit for detection and identification of swine fever virus field strains and vaccine strains in pig umbilical cord blood
[0120] 1. Validation of amplification efficiency
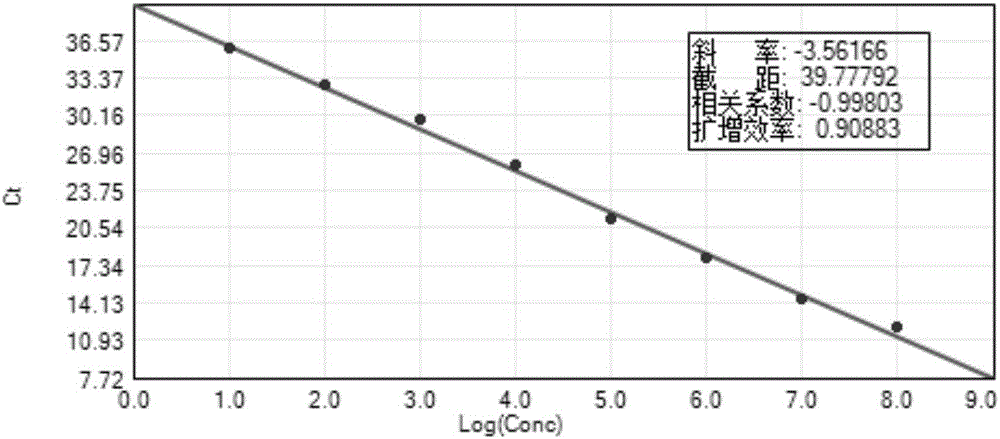
[0121] Perform a 10-fold serial dilution of the positive control clone plasmid pEASY-5UTR2 to make its copy number: 10 8 -10 1 copies / μl, each gradient was repeated three times for real-time fluorescent RT-PCR, and a standard curve was made according to the amplification results.
[0122] figure 1 It is the gradient amplification standard curve (FAM channel) of 10-fold dilution of the positive control of the present invention, wherein the parameters of the standard curve are as follows: slope: -3.56, intercept: 39.78, correlation coefficient: 0.998, amplification efficiency: 0.91.
[0123] figure 2 In order to invent a 10-fold diluted gradient amplification standard curve (VIC channel) for the positive control, the parameters of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com