Method for synthesizing 3-hydracrylic acid ester
A technology of hydroxypropionate and cobalt carbonyl, which is applied in the field of synthesizing 3-hydroxypropionate, can solve the problems such as difficult separation of catalysts, and achieve the effect of mild catalytic reaction conditions, high activity and difficult separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Catalyst preparation
[0040] 1.0 mmol of Co 2 (CO) 8 Dissolve in 740mmol of methanol, add poly(4-vinylpyridine) powder with a number average molecular weight of 100,000, make the molar ratio of nitrogen to cobalt carbonyl 2:1, stir at room temperature for 2 hours to obtain a catalyst mixture.
[0041] 2. Synthesis of methyl 3-hydroxypropionate
[0042] The obtained catalyst mixture was transferred to a 100 mL reactor; the reactor was purged three times with nitrogen, and carbon monoxide and 50 mmol ethylene oxide were added to make the system pressure 5 MPa; reacted at 60° C. for 6 hours. After the reaction was completed, the reactor body was cooled to 0° C., the pressure was slowly released to normal pressure, and the reactor was purged three times with nitrogen. Sampling analysis showed that the conversion rate of ethylene oxide was 31%, and the selectivity of methyl 3-hydroxypropionate was 79%.
[0043] For the convenience of comparison, the results of the sy...
Embodiment 2
[0045] 1. Catalyst preparation
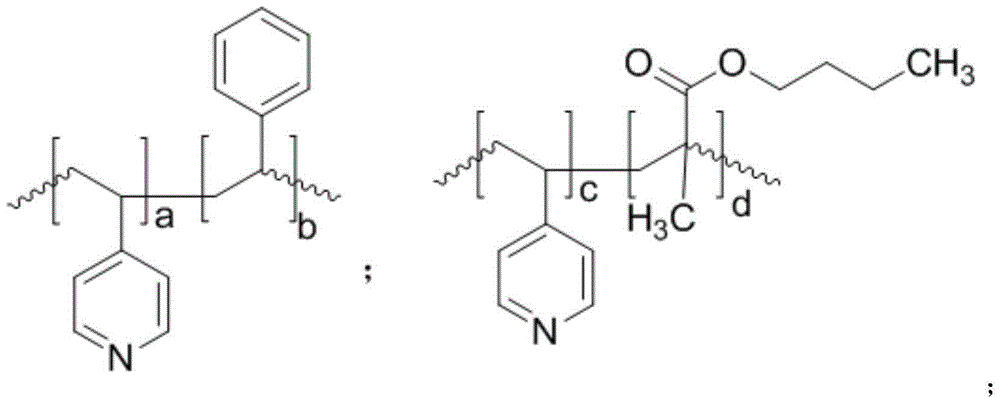
[0046] 1.0 mmol of Co 2 (CO) 8 Dissolve in 740mmol of methanol, add poly(4-vinylpyridine-co-styrene) powder with a number average molecular weight of 100,000 (wherein the molar ratio of 4-vinylpyridine monomer units to styrene monomer units is 1:1), The molar ratio of nitrogen to cobalt carbonyl was 2:1, and stirred at room temperature for 2 hours to obtain a catalyst mixture.
[0047] 2. Synthesis of methyl 3-hydroxypropionate
[0048]The obtained catalyst mixture was transferred to a 100 mL reactor; the reactor was purged three times with nitrogen, and carbon monoxide and 50 mmol ethylene oxide were added to make the system pressure 5 MPa; reacted at 60° C. for 6 hours. After the reaction was completed, the reactor body was cooled to 0° C., the pressure was slowly released to normal pressure, and the reactor was purged three times with nitrogen. Sampling analysis showed that the conversion rate of ethylene oxide was 34%, and the selectivi...
Embodiment 3
[0051] 1. Catalyst preparation
[0052] 1.0 mmol of Co 2 (CO) 8 Dissolve in 740mmol of methanol, add poly(4-vinylpyridine-co-ethylvinylbenzene) powder with a number average molecular weight of 100,000 (wherein the molar ratio of 4-vinylpyridine monomer unit to ethylvinylbenzene monomer unit 1:1), the molar ratio of nitrogen to cobalt carbonyl was 2:1, and stirred at room temperature for 2 hours to obtain a catalyst mixture.
[0053] 2. Synthesis of methyl 3-hydroxypropionate
[0054] The obtained catalyst mixture was transferred to a 100 mL reactor; the reactor was purged three times with nitrogen, and carbon monoxide and 50 mmol ethylene oxide were added to make the system pressure 5 MPa; reacted at 60° C. for 6 hours. After the reaction was completed, the reactor body was cooled to 0° C., the pressure was slowly released to normal pressure, and the reactor was purged three times with nitrogen. Sampling analysis showed that the conversion rate of oxirane was 40%, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com