Application of PreS1 in preparation of hepatitis B vaccine and treatment of chronic hepatitis B
A technology for chronic hepatitis B and hepatitis B virus, applied in the field of biomedicine, can solve the problem that HBp vaccine has no good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
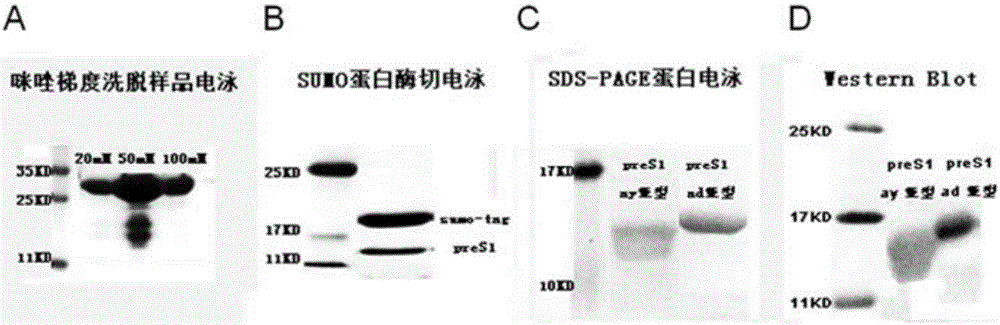
[0036] Example 1: Expression and purification of the vaccine
[0037] Using AAV-HBV1.3 (purchased from Beijing Wujiahe Institute of Molecular Medicine Co., Ltd.) infected mouse liver cDNA (obtained by extracting tolerant mouse liver tissue total RNA and reverse transcription) as a template, design specific primers, The HBV PRES1 (ay subtype) DNA fragment was amplified by PCR reaction, and its nucleotide sequence is:
[0038] ATGGGGCAGAATCTTTCCACCAGCAATCCTCTGGGATTCTTTCCCGACCACCAGTTGGATCCAGCCTTCAGAGCAAACACAGCAAATCCAGATTGGGACTTCAATCCCAACAAGGACACCTGGCCAGACGCCAACAAGGTAGGAGCTGGAGCATTCGGGCTGGGTTTCACCCCACCGCACGGAGGCCTTTTGGGGTGGAGCCCTCAGGCTCAGGGCATACTACAAACTTTGCCAGCAAATCCGCCTCCTGCCTCCACCAATCGCCAGACAGGAAGGCAGCCTACCCCGCTGTCTCCACCTTTGAGAAACACTCATCCTCAGGC C (SEQ ID NO: 1)
[0039] Its amino acid sequence is:
[0040] MGQNLSTSNPLGFFPDHQLDPAFRANTANPDWDFNPNKDTWPDANKVGAGAFGLGFTPPHGGLLGWSPQAQGILQTLPANPPPASTNRQTGRQPTPLSPPLRNTHPQA (SEQ ID NO: 2)
[0041] In addition, the DNA sequence of HBV PRES1 (ad subt...
Embodiment 2
[0050] Example 2: The preS1 antigen was used as a vaccine to test its effect of inducing an immune response in the body.
[0051] Use 10μg of the preS1 protein obtained in Example 1 (alone or in combination with the following adjuvants: aluminum adjuvant (purchased from InvivoGen, 1:1-1:9 mix), 10μg TLR-4 ligand MPLA adjuvant (purchased From InvivoGen), 30μgTLR-9 ligand CpG adjuvant (synthesized by Invitrogen) or Freund’s adjuvant (IFA) (purchased from Sigma) subcutaneously immunized C57BL / 6 mice (6-8 weeks, male, purchased from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd.). The immunization process is as follows figure 2 Shown in A. After immunization, the anti-preS1 antibody response in the serum was detected by ELISA ( figure 2 B); Detection of preS1-specific T cell immune response in the spleen by ELISPOT ( figure 2 C). The experimental results show that preS1 protein combined with adjuvant can induce strong antibody response and specific T cell immune res...
Embodiment 3
[0052] Example 3: Through HBV in vivo infection experiment, the ability of preS1 antigen vaccine to prevent HBV infection was verified.
[0053] By the immunization method in Example 2, 10 μg of the preS1 protein purified in Example 1 was combined with Freund’s adjuvant to subcutaneously immunize C57BL / 6 mice (6-8 weeks, male, purchased from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd. the company). The immunization process is like image 3 As shown in A, two subcutaneous immunizations were performed at an interval of 14 days. High titer of preS1 specific antibody anti-preS1 ( image 3 B). Then infect 1x10 through the tail vein 10 vg (viral genome) AAV-HBV1.3 (purchased from Beijing Wujiahe Institute of Molecular Medicine Co., Ltd.), blood is collected every week after infection to detect HBV-related antigens in serum, including HBsAg antigen, preS1 antigen and HBV-DNA changes .
[0054] The results show that compared with the group without protein immunization, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com