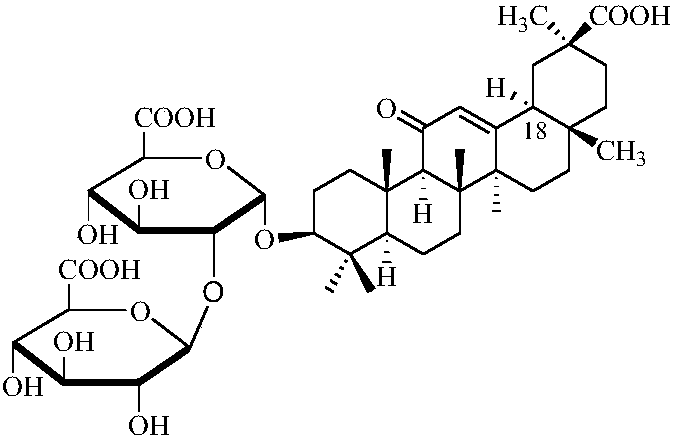
A kind of preparation method of 18α type glycyrrhizic acid diammonium salt
A technology of diammonium glycyrrhizinate and monoammonium glycyrrhizinate, which is applied to the preparation of sugar derivatives, chemical instruments and methods, steroids, etc. High, unrealistic acquisition and other problems, to achieve the effect of excellent color, high yield and content, and reduce refining steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A preparation method of 18α type diammonium glycyrrhizinate, comprising the following steps:
[0025] (1) Take 1kg of monoammonium glycyrrhizinate, 5L of water and 2.5kg of sodium hydroxide as raw materials, add the required amount of water to monoammonium glycyrrhizinate, first add 750g of sodium hydroxide, and after fully dissolving, add The remaining amount of sodium hydroxide was stirred and reacted for 7.5 hours while heating at 80°C;
[0026] (2) After stopping heating, add 5mol·L -1 hydrochloric acid solution to adjust the pH to 3, and extract with 0.5 times the amount of n-butanol to obtain an organic layer;
[0027] (3) Take a small amount of organic layer and carry out liquid chromatography detection to determine whether the configuration transformation is complete. The detection result configuration transformation is relatively complete, and the content of 18α-glycyrrhizic acid is 92.5%;
[0028] (4) The configuration conversion is relatively complete, and ...
Embodiment 2
[0030] A preparation method of 18α type diammonium glycyrrhizinate, comprising the following steps:
[0031] (1) Take 1 kg of monoammonium glycyrrhizinate, 6 L of water and 3 kg of sodium hydroxide as raw materials, add the required amount of water to monoammonium glycyrrhizinate, first add 1000 g of sodium hydroxide, and after fully dissolving, add the remaining A certain amount of sodium hydroxide was stirred and reacted for 6 hours while heating under the condition of 85°C;
[0032] (2) After stopping heating, add 6mol·L -1 hydrochloric acid solution to adjust the pH to 3, and extract with 0.5 times the amount of n-butanol aqueous solution to obtain an organic layer;
[0033] (3) Take a small amount of organic layer and carry out liquid chromatography detection to determine whether the configuration conversion is complete. The detection result configuration conversion is relatively complete, and the content of 18α-glycyrrhizic acid is 93.5%;
[0034] (4) The configuration...
Embodiment 3
[0036] A preparation method of 18α type diammonium glycyrrhizinate, comprising the following steps:
[0037] (1) Get 1kg of monoammonium glycyrrhizinate, 6.5L of water and 3.5kg of sodium hydroxide as raw materials, add required amount of water in monoammonium glycyrrhizinate, first add 1500g of sodium hydroxide, after dissolving fully, then The remaining amount of sodium hydroxide was added, and the reaction was stirred while heating at 80°C for 6h;
[0038] (2) After stopping heating, add 6mol·L -1 hydrochloric acid solution to adjust the pH to 3, and extract with 0.5 times the amount of ethyl acetate to obtain an organic layer;
[0039] (3) Take a small amount of organic layer and carry out liquid chromatography detection to determine whether the configuration conversion is complete. The detection result configuration conversion is relatively complete, and the content of 18α-glycyrrhizic acid is 93.0%;
[0040] (4) The configuration transformation is relatively complete, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com