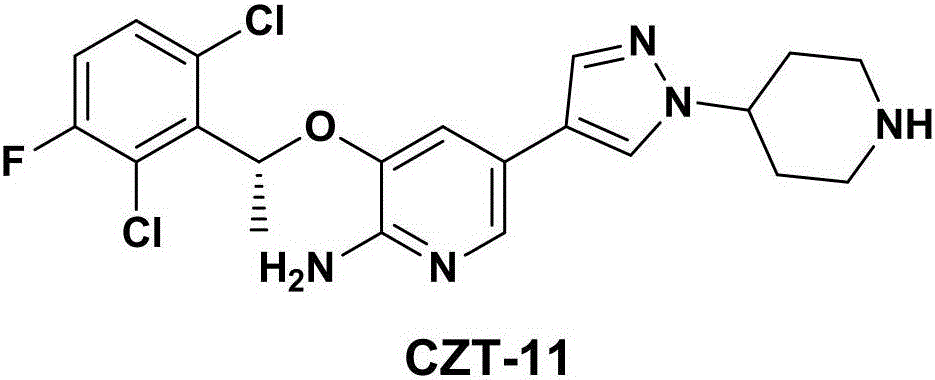
Crizotinib intermediate, preparation method and crizotinib preparation method
A technology of crizotinib and intermediates, applied in the field of chemical preparation, can solve the problems of long reaction cycle, unfavorable industrial production, complex reaction, etc., and achieve the effect of reduced production cost, favorable for large-scale industrial production, and simple purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
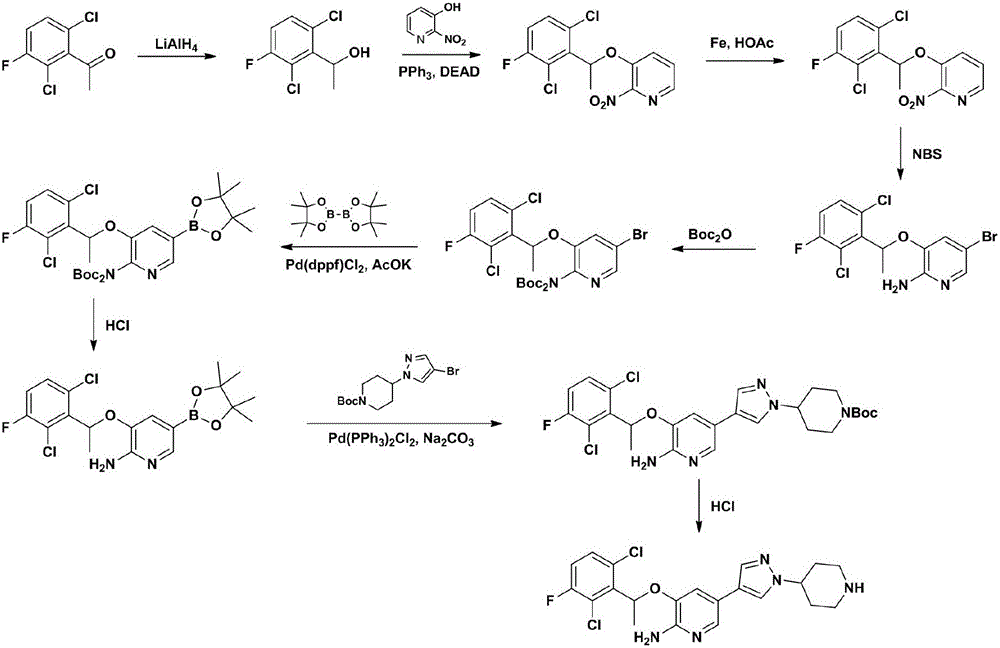
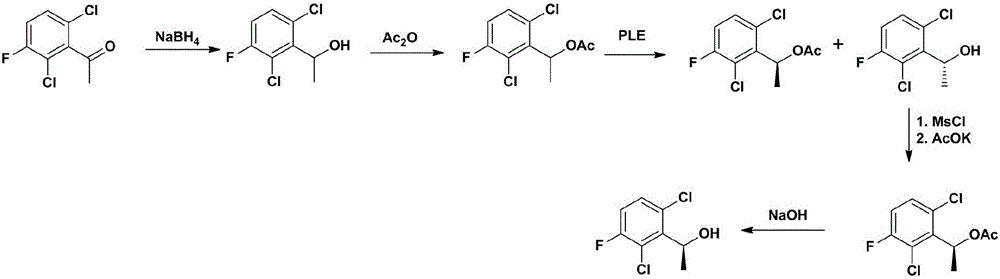
Examples
Embodiment 1
[0048] Embodiment 1, the preparation of compound (CZT-2);
[0049] Dissolve 2.07 kg of compound (CZT-1) and 120 g of L-proline in 20 liters of dry absolute ethanol, cool down to 0°C, add 900 g of sodium borohydride slowly in batches, and keep at 0°C after the addition is complete React for 5 hours. After filtration and concentration, 2.1 kg of crude product was obtained, and 1.82 kg of oil (CZT-2) was obtained by distillation under reduced pressure, with a yield of 87% and an e.e value greater than 99%.
Embodiment 2
[0050] Embodiment 2, the preparation of compound (CZT-3);
[0051] 1.82 kg of compound (CZT-2), 1.23 kg of 3-hydroxy-2-nitropyridine and 2.60 kg of triphenylphosphine were dissolved in 15.0 liters of dry toluene, nitrogen protection, and cooled to -20 degrees. Slowly add 2.04 kg of DIAD in 2.62 liters of toluene solution within 3 hours, keep the system temperature between -20°C and -10°C, and slowly raise the temperature to 25°C for 2 hours after the addition is complete. Add 0.16 L of water to quench the reaction, cool to -5°C, filter, and concentrate the filtrate under reduced pressure. Recrystallized with 7.5 liters of absolute ethanol to obtain 2.50 kg of compound (CZT-3), with a yield of 85%.
Embodiment 3
[0052] Embodiment 3, the preparation of compound (CZT-4);
[0053]Dissolve 2.45 kg of compound (CZT-3) in 24.5 liters of absolute ethanol and 24.5 liters of glacial acetic acid, add 2.04 kg of iron powder, react at room temperature for 5 hours, filter with diatomaceous earth, and wash the filter residue with 10 liters of ethyl acetate. Add 75 liters of ethyl acetate in the filtrate, successively with the water of 50 liters * 3, the sodium bicarbonate solution of 50 liters * 2 and the saturated sodium chloride solution washing of 50 liters, filter after drying, concentrate under reduced pressure to obtain 2.35 kilograms of compounds ( CZT-4), yield 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com