Gemcitabine ProTide hypoxic activation prodrug and application thereof
A technology of gemcitabine and oxygen activation, which is applied in organic chemistry, antineoplastic drugs, drug combinations, etc., can solve the problem of limited tumor effect, and achieve the effects of reducing toxic and side effects, excellent anticancer effect, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Synthesis of 3′-O-(tert-butoxycarbonyl) gemcitabine
[0021] Synthetic route (obtained by reference method, The Journal of Organic Chemistry, 1999, 64: 8319-8322):
[0022]
[0023] Experimental operation: gemcitabine (gemcitabine, 0.60g, 2mmol), Na 2 CO 3 (1.06 g), 40 mL of dioxane, 40 mL of water, and di-tert-butyl dicarbonate (DBDC, 0.44 g, 2 mmol) were stirred at room temperature for 48 hours. Add 20mL water, extract with 2×300mL ethyl acetate, Na 2 SO 4 Dry and concentrate under reduced pressure. Flash column chromatography (CH 2 Cl 2 -Ethyl acetate-EtOH 1:1:0.02) to give 3'-O-(N-tert-butoxycarbonyl) gemcitabine (0.60 g). 1 H NMR(DMSO-d6,300MHz)δ(ppm): 7.64(d,1H)7.40(d,2H)6.21(t,1H)5.81(d,1H)5.25-5.12(m,2H),4.13(t ,1H)3.71-3.60(m,2H)1,45(s,9H).
Embodiment 2
[0024] Example 2: Synthesis of Compound 001
[0025] Synthetic route (obtained by reference method, The Journal of Medicinal Chemistry, 2014, 57:1531-1542):
[0026]
[0027] Add 10mL of redistilled dichloromethane to a 100mL eggplant-shaped flask, add phosphorus oxychloride (0.2g, 1.3mmol) in the dichloromethane, place the reaction system at -78℃, and add triethylamine to it (0.13g, 1.3mmol), after stirring for 15min, add dropwise a solution of phenol (0.153g, 1mmol) in dichloromethane (5mL) for 15min, react at -78°C for 1h, and at room temperature for 1h. Continue to place the entire reaction system at -78°C, add 0.23g (2.3mmol) of triethylamine to it, stir for 15min, add 0.275g (1mmol) of L-alanine 1-(4-nitrate) 10 mL of distilled dichloromethane solution of phenyl)ethanol ester hydrochloride and stirred for 3h. Take another 50mL eggplant-shaped bottle, add 30mL redistilled dichloromethane, add 0.29g (0.8mmol) 3′-O-(tert-butoxycarbonyl) gemcitabine, add 0.2g (2mmol) triethylam...
Embodiment 3
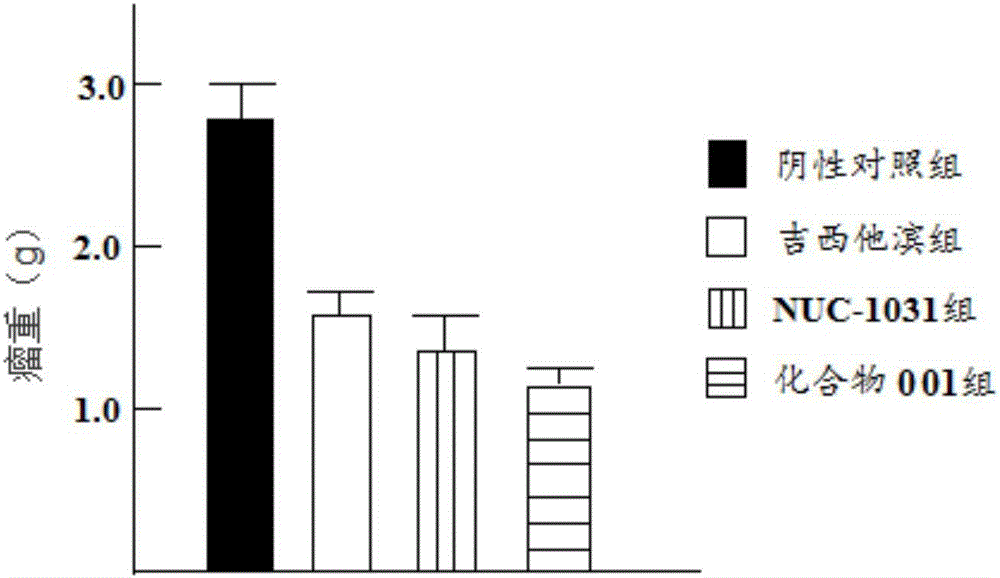
[0043] Example 3: Study on the in vitro inhibitory effect of the target compound on tumor cell proliferation under normoxia and hypoxia
[0044] Take logarithmic growth phase tumor cells, add 0.25% trypsin to digest for 3 minutes, suspend the cells with RPMI-1640 containing 10% calf serum, count, and adjust the cell concentration to 1×10 5 Pcs / mL, inoculate 100μL / well in a 96-well cell culture plate special for Top-count, 37℃, 5% CO 2 Incubate for 24h. Then divide the cells into the experimental group and the control group. The experimental group is added with the target compound solution (0.001μg / mL, 0.01μg / mL, 0.1μg / mL, 1μg / mL, 10μg / mL), each concentration is four duplicate holes , And the volume of each well is made up to 200μL. After adding samples, each group continued to cultivate for 72h (hypoxic group under 5% CO 2 , 95% N 2 Continue to incubate for 72h respectively), before the end of the culture, add to each well 3 H-TdR 3×10 5 Bq, use Top-count to measure the CPM (coun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com