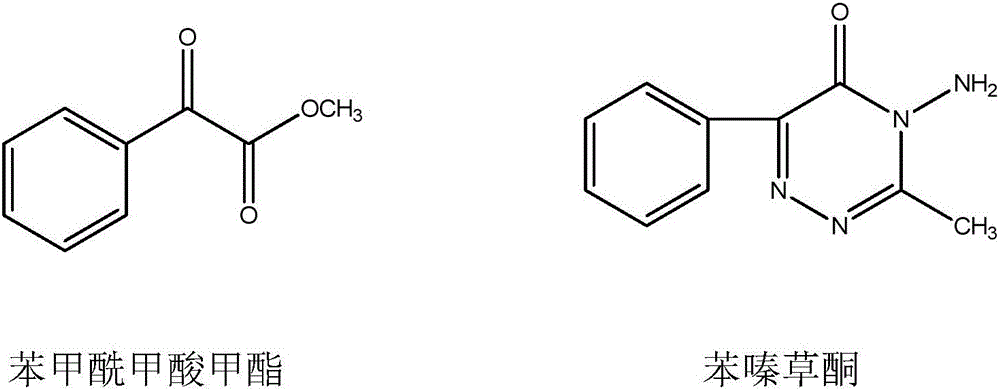
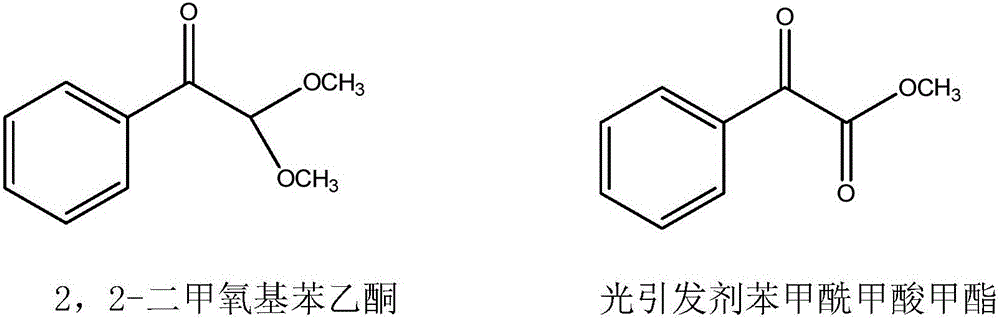
New synthesis technology of methyl benzoylformate
A benzoylformic acid, a new process technology, applied in the field of compound synthesis, can solve the problems of high risk, heavy environmental pollution, high production cost, etc., and achieve the effect of cheap price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
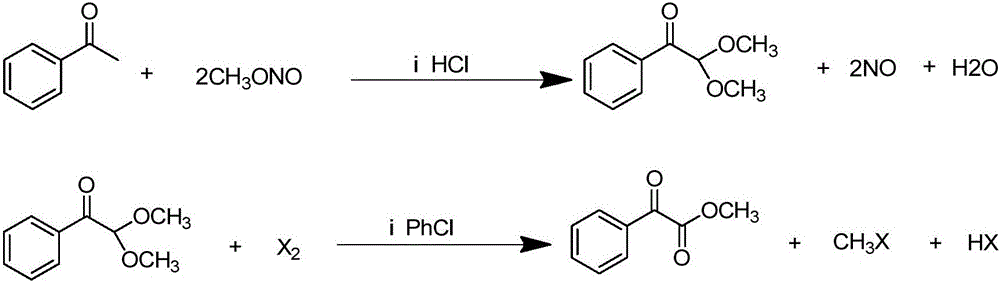
[0029] (1) In a 2000-liter reactor, add 600kg of methanol and 240kg of acetophenone, feed 64kg of dry hydrogen chloride gas under ice water cooling, and feed 260kg of methyl nitrite at a temperature of 30-35°C. After passing through, continue React at this temperature for 2 hours, stop the reaction, steam out methanol to obtain the crude product, add 400kg of toluene, neutralize with 10% sodium hydroxide solution, separate the liquids to obtain the toluene solution in the upper layer, and steam the toluene to obtain the crude product. The product was subjected to vacuum distillation to obtain 248kg of 2,2-dimethoxyacetophenone with a yield of 68.9%.
[0030] (2) In a 1000-liter reactor, add 180kg of 2,2-dimethoxyacetophenone, 18kg of 4-methyl-2,6-di-tert-butylphenol and 400kg of chlorobenzene, heat to 110°C, and feed Chlorine 71kg, have hydrogen chloride and methyl chloride gas to emit in the reaction process, gas chromatographic tracking reaction process, stop feeding chlorin...
Embodiment 3
[0032] In a 1000ml reaction flask, add 400g of cyclohexane, 18g of 4-methyl-2,6-di-tert-butylphenol and 180g of 2,2-dimethoxyacetophenone, heat to 60-70°C, drop 158g bromine, produce methyl bromide and hydrogen bromide gas in the reaction process, continue stirring reaction for 1 hour after dripping, stop reaction, cool, pass into nitrogen to get rid of excess bromine and hydrogen bromide gas, use saturated carbonic acid Washing with sodium solution, separating the liquids, distilling off the cyclohexane to obtain a crude product, and rectifying under reduced pressure to obtain 152 g of methyl benzoylformate with a yield of 92.7%.
Embodiment 4
[0034] In a 1000ml reaction flask, add 400g of chlorobenzene, 18g of 4-methyl-2,6-di-tert-butylphenol and 180g of 2,2-dimethoxyacetophenone, heat to 110-120°C, add dropwise 158g Bromine, methyl bromide and hydrogen bromide gas are produced during the reaction process, continue to stir the reaction for 1 hour after the drop is over, stop the reaction, cool, feed nitrogen to drive away the excess bromine and hydrogen bromide gas, and use saturated sodium carbonate The solution was washed and separated, and the chlorobenzene solution in the lower layer was distilled off under reduced pressure to obtain a crude product, which was rectified under reduced pressure to obtain 148 g of methyl benzoylformate, with a yield of 90.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com