A pharmaceutical composition containing rhil-12 for treating inactive hepatitis B
A composition and active technology, applied in the field of biological preparations, can solve the problems of inability to form immune memory, inability to completely remove, and inability to remove HBV well, so as to improve acquired immune function, enhance cellular immunity, and improve Effects of cellular and humoral immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
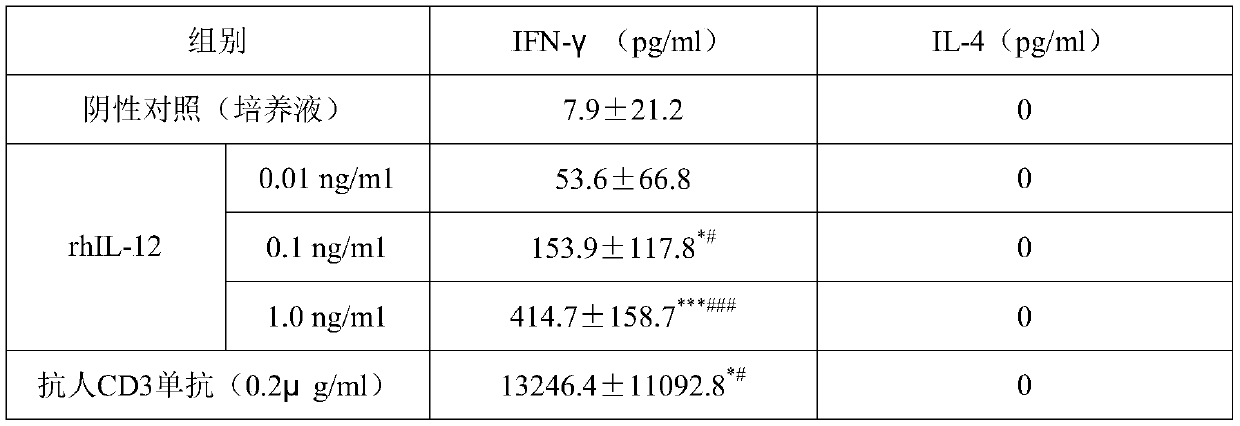
[0020] Example 1 The effect of rhIL-12 on the induction of IFN-γ by PBMCs of healthy people and CHB patients
[0021] 1. Experimental data: rhIL-12 in this example was purchased from Guangzhou Kaitai Biotechnology Co., Ltd.; human IFN-γELISA kit was purchased from BD / Pharmingen (Becton Dickinson / Phar Mingen USA); RPMI-1640 culture medium , glutamine and Hanks solution were purchased from GIBCO (USA); dextran Ficoll-Hypaque was purchased from Shanghai Huajing Biotechnology Company; fetal bovine serum was purchased from GIBCO.
[0022] 20 cases of healthy people and 62 cases of CHB patients were screened (including 21 cases in the immune tolerance phase, 22 cases in the immune clearance phase, and 19 cases in the inactive HBsAg carrier phase). Among them, the selection criteria for healthy people: HBsAg negative, HBsAb positive. All hepatitis B patients were not combined with hepatitis C infection, and all subjects were aged 15-55, regardless of gender.
[0023] 2. Test method...
Embodiment 2
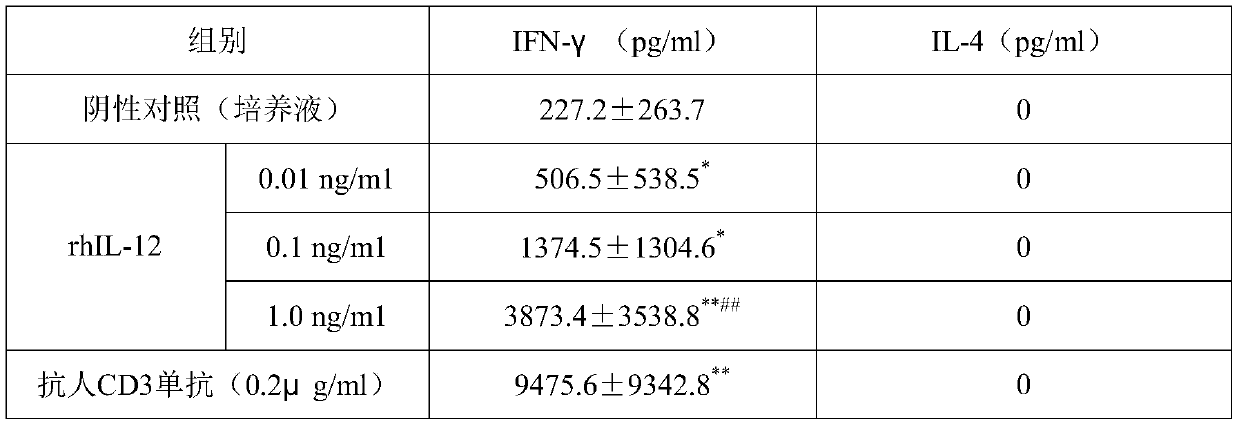
[0043] Example 2 Effects of combined application of rhIL-12 and specific antigens on the production of IFN-γ in BALB / c mice
[0044] 1. Experimental data: rhIL-12 in this example was purchased from Guangzhou Kaitai Biotechnology Co., Ltd.; RPMI1640 basal culture medium and FBS were purchased from GIBCO (USA); Ficoll lymphocyte separation medium was purchased from Shanghai Huajing Biotechnology company.
[0045] 40 clean-grade BALB / c mice, half male and half female, weighing 20 g, were purchased from the Experimental Animal Center of Southern Medical University.
[0046] 2. Test method:
[0047] 40 mice were randomly divided into normal saline group (normal saline 150 μl / mouse), rHBsAg group (10 μg / mouse), Mycobacterium vaccae group (10 μg / mouse), rhIL-12 group (0.3 μg / mouse), rHBsAg (10 μg / monkey)+Mycobacterium vaccae (10 μg / bird)+rhIL-12 (0.3 μg / bird) group, a total of five groups, 8 rats in each group, half male and half male. Dosing twice, and dosing again after 72 hours...
Embodiment 3
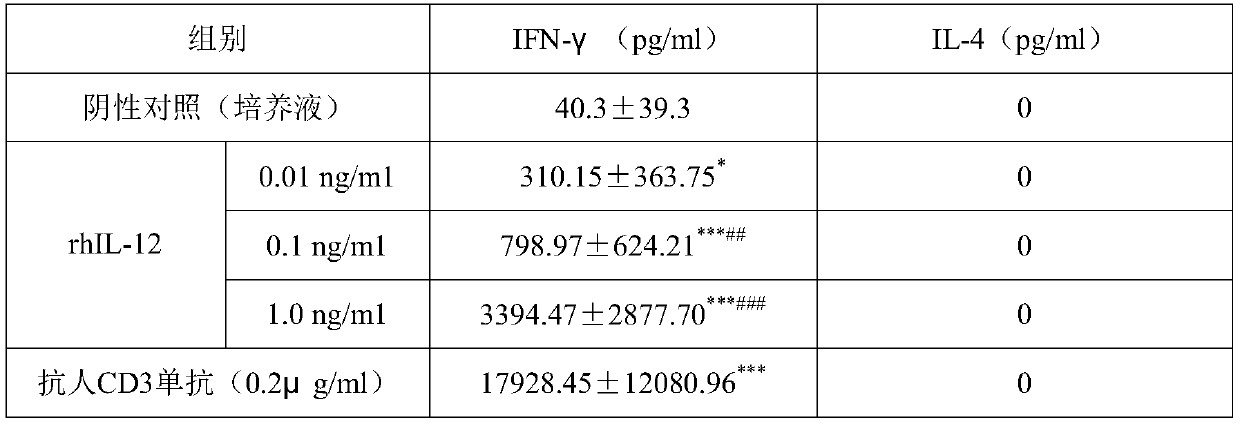
[0055] Example 3 The combined application of rhIL-12 and specific antigen on the production of IFN-γ by PBMCs of healthy people and CHB patients
[0056] 1. Experimental data: rhIL-12 in this example was purchased from Guangzhou Kaitai Biotechnology Co., Ltd.; rHBsAg was purchased from Shenzhen Kangtai Biotechnology Co., Ltd.; Mycobacterium vaccae for injection was purchased from Anhui Zhifeilong Kema Biopharmaceutical Co., Ltd. Ltd.; human IFN-γELISA kits were purchased from BD Company.
[0057] Screening 15 cases of chronic hepatitis B patients, aged 17-49 years, with an average of 31.17 years old. Acute and chronic diseases caused by hepatitis A virus (HAV), hepatitis C virus (HCV), hepatitis D virus (HDV), hepatitis E virus (HEV) infection and other causes (drugs, alcohol, poisoning) were excluded for all patients Liver damage. Another 9 healthy volunteers were selected as controls, all of whom had been vaccinated with recombinant HBsAg vaccine, except for HBsAb positive...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com